7. Control of fungal morphogenesis
The examples I have discussed so far indicate that expression of the genes which contribute to morphogenesis relies on previously-formed cell and tissue structures. Further, developmental gene expression depends on the environment as this provides the 'context' or 'network' within which new gene products must work. This 'context' includes chemical, electrical and structural or mechanical tensions as well as cell and organelle structures.
Filamentous fungi must be unique in offering, as part of the normal pattern of growth of one organism, the opportunity to study the behaviour of independent cells undergoing vegetative growth (that is, as the mycelium), as well as the behaviour of cells which are contributing to the structure and development of complex organs and tissues (that is, in multicellular structures like fruit bodies and sclerotia). An essential preliminary to understanding how these events might be controlled is to appreciate the nature of ‘cells’ in filamentous fungi. The free-living microbial cell shows great flexibility and adaptability in responding to its environment but the contact with the environment is so close that the concept of the behaviour of the cell has no meaning unless the whole system of cell plus environment is considered. This is not the case for the cells of a tissue where the organisation and metabolism of the cells which constitute the tissue can in large measure determine the environment of individual cells. However, the contribution to a tissue must be viewed as carrying with it some loss of freedom of response - a diminution of the flexibility and adaptability of which the individual cell may be capable - in favour of the concerted action required of the tissue as a whole. In most organisms the behaviour of independent cells can only be studied under the most artificial conditions by investigation of cell cultures. For the fungi the vegetative growth of 'undifferentiated' cells in the form of a mycelium is a normal part of the life cycle. Yet that same mycelium may give rise to fruiting bodies which are as complex in structure and every bit as massive (some Zambian mushrooms can approach one metre in cap diameter) as are the organs and tissues of higher organisms (Moore, 1984b).
Such uniqueness, though, does bring with it some difficulties. One such has already been encountered by the use of the word 'cell' in paragraphs above. The fungal hypha is a filament composed of numerous compartments connected end to end, yet hyphal growth is so highly polarised that true extension growth is absolutely limited to the hyphal tip, so the whole morphology of the hypha depends on events taking place at its apex. Fungal hyphae are as variable between species as is any other aspect of fungal biology, but generally speaking the hyphal filament, when separated into compartments by cross-walls, has an apical compartment which is perhaps up to ten times the length of the subterminal or intercalary compartments. The majority of the lower fungi have coenocytic hyphae (generally lacking septa); but then the lower fungi do not form multicellular structures. Fungal primary septa are formed, always at right angles to the long axis of the hypha, by a constriction process in which a peripheral belt of microfilaments interacts with microvesicles and other membranous cell organelles (Moore, Robson & Trinci, 2011; see pp. 92 et seq. and 144 et seq. for a full account). The septa which come to divide hyphae into compartments may be complete (imperforate) or perforated by a large central pore. The latter may be open (and offer little apparent hindrance to the passage of cytoplasmic organelles and nuclei); or may be plugged with Woronin bodies in Ascomycota; or protected by a complex cap structure (the parenthesome apparatus) derived from the endoplasmic reticulum (the dolipore septum of Basidiomycota); in any of these cases movement of cytoplasm and cytoplasmic components between adjacent compartments is under effective control. Septal form may be modified by the hyphal cells on either side of the septum, and may vary according to age, position in the mycelium, or position in the tissues of a differentiated structure.
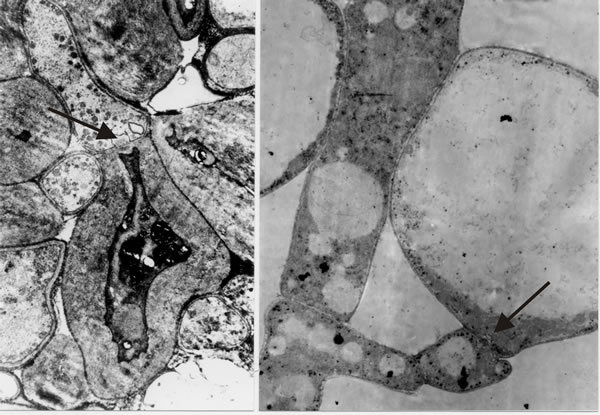
It is the open hyphal septum that presents us with the philosophical problem: if a cell is a closed container, do fungal septa divide a hypha into cells that each experience an individual existence, or do fungal septa divide a hypha into mere compartments that share (by free movement of the cytoplasm) the concerted experience of the whole hypha? I hedged my bets to begin with by combining the terms and referring to “discrete compartments; whether those compartments are in free cytoplasmic contact with one another, and whether they are uninucleate or multinucleate, will vary with the situation” (Moore, 1984b). However, as we undertook more and more detailed microscopic analyses and observed more and more extremes of differentiation occurring across hyphal septa (Fig. 30), I became more and more convinced that hyphae are true multicellular structures.
The hypha is subdivided into discrete cells, each of which experiences an individual existence by controlling exchanges across the hyphal septa to the same degree of sophistication that an independent single cell belonging to any of the eukaryote kingdoms controls its exchanges with the outside world. The multicellular nature of the filamentous fungal hypha cannot be doubted, at least to the extent of its being separated into compartments whose interactions are carefully regulated and which can exhibit contrasting patterns of differentiation (Fig. 30); indeed, the ancestral formation of the septate hypha can be interpreted as the deep evolutionary origin of multicellularity (Moore, 2013). Furthermore, in mycelial fungi, formation of hyphal branches is the only way to increase the number of growing points and this means that in filamentous fungi hyphal branching is the equivalent of cell division in animals and plants. Kinetic analyses show clearly that filamentous fungal growth can be interpreted on the basis of a regular cell cycle (Moore, Robson & Trinci, 2011; see chapter 4). Thus, filamentous fungi must be considered to be cellular organisms producing differentiated tissues composed of specialised cell communities
which are the progeny of an initial cell or cell population which is induced to start differentiation.
|
Fig. 30. Images that convinced me that fungal hyphae are subdivided into true cells. Transmission electron micrographs of differentiation across the dolipore septum in Coprinopsis cinerea. At left is a section of the medulla of a sclerotium in which thick-walls and thin-walls occur in hyphal compartments either side of a septum (arrow). At right is a section of an immature hymenium in which the enormously inflated paraphysis shares a dolipore septum (arrow) with a cell below which is of more normal hyphal appearance. Images modified from Moore (1998a). |
Although fungi have been used as experimental organisms for research in biochemistry and molecular genetics (including cell differentiation), their multicellular structures contribute little to the main stream of research in developmental biology; indeed, I suspect that many people, including some mycologists, would deny that fungi exhibit a developmental biology. Yet fungi produce some extremely complex structures and their study could contribute a great deal to our understanding of developmental processes. Analysis of cell differentiation and pattern formation processes can reveal general strategies and conserved pathways, as well as alternative mechanisms. In a more practical sense, many fungal pathogens employ multicellular structures in their infection and survival strategies, whilst the commercial value of cultivated fungi obviously depends on their morphogenetic ability; understanding the architecture and construction of multicellular structures may lead to improved control and cropping methods (Moore, 1988).
Plants, animals and fungi are now seen as three quite distinct kingdoms of eukaryotic organisms which must have separated in evolution at some level long before the multicellular grade of organisation was established (Moore, 2013). Parallels between the morphogenesis of fungi and that of other eukaryotic organisms are worth seeking so that we can make use of the conceptual framework which has already been established in the hope of developmental models which can spur fresh experimentation with fungal material. When this is done and due allowance made for the peculiarities imposed by the unique aspects of fungal structural organisation, remarkable similarities in the orchestration of morphogenesis emerge between fungi, plants and animals (Moore, 1988). That there should be differences between developmental phenomena in fungi, plants and animals comes as no great surprise; that there should be such profound similarities as seem to exist is little short of amazing. There is no doubt that, in all three groups, multicellularity evolved long after the three evolutionary lines had diverged into their characteristic and totally separate modes of form, structure and behaviour. Thus, little, if any at all, of the organisation which permits multicellular morphogenesis could have been possessed by any common ancestor (Moore, 2013). The parallels which do seem to exist in the basic regulation of morphogenesis in plants, animals and fungi imply convergent evolution; but further, and perhaps more interestingly, imply that there is only this limited number of ways of solving the problems associated with organising populations of cells into particular patterns irrespective of the nature of those cells. The rules which govern morphogenesis appear to be natural laws owing more to the physical and chemical phenomena involved than to the biological entities that respond to them (Moore, 1988). Homologues and analogues of all of the developmental mechanisms known in animals and plants can be found in fungi, including:
- mechanical effects;
- temporal sequencing;
- pattern formation and morphogenetic fields;
- programmed cell death [view page];
- reaction with an extracellular matrix [view page].
Mechanical effects. The role of mechanical stresses in producing the stem structure is one example already discussed. Another example is the regular radial arrangement of gills. In both Coprinopsis cinerea and Volvariella volvacea embryonic gills are contorted (Chiu & Moore, 1990a; Chiu & Moore, 1990b). In both species the first-formed gills were radially arranged, but as the cap expands more gills are formed. In V. bombycina, new gills were formed in two ways (Chiu & Moore, 1990a). First, by bifurcation of an existing gill near its free edge. Initiation of the folding which produced bifurcations on existing gills was localised and irregular, resulting in sinuous, contorted gills. The formation of two daughter gills depended on completion of the bifurcation along the entire edge of the parental gill. New generations of gills appeared as ridges in the region between existing gill roots, creating new folds on the cap context representing the free edges of new secondary or tertiary gills, the gill spaces on either side extending into the cap context as the gill grew by its root differentiating from the context (Moore,1987).
The sinuous 'embryonic' gills of a Volvariella bombycina fruit body are inflated into their final shape by the inflation of the cells of the trama. The hymenium of V. bombycina is a skin-like layer of tightly appressed cells, and the trama of the gill becomes filled with greatly inflated cells as maturation proceeds. These features suggest that expansion of tramal cells in gills enclosed by the hymenial 'epidermis' generates compression forces which effectively inflate, and so stretch, the embryonic gills to form the regularly radial pattern of the mature cap. When complete, this stressed-skin construction exhibits great similarity to the stretched skin constructions which is used for light-weight, high-strength engineering structures.
In Coprinopsis cinerea, more gills are added as the fruit body enlarges by bifurcation of existing gills either on one side or at the stem-gill junction, and by division of gill organisers at the roots of existing gills (explained below [view now]). Consequently, Coprinopsis gills are also formed as convoluted plates. Subsequently, tensions generated by growth of other parts of the fruit body place geometrical stress on the 'embryonic' gills, like a folded cloth being straightened by stretching. Such a mechanism requires that the folded elements (in this case the gills) are anchored. The connection of primary gills to the stem provides the initial anchorage; subsequently cystidium-cystesium pairs interconnect gill plates around the stem. Tensions generated by expansion of the cap will then be communicated and balanced throughout the structure. Cystidium-cystesium pairs act as tension elements whose function is to hold adjacent hymenia together as cap expansion pulls the gills into shape. The strength of the adhesion between cystidia and the opposing hymenium (see discussion on p. 486 in Horner & Moore, 1987) is essential to this mechanical function; when they do not exist, as in the revoluta mutant (Chiu & Moore, 1990b). This is why cystidia must be thought of as tension elements and not buttresses. An engineering comparison here, would be the bracing (tensioning) wires which are strung between the wings of a biplane and which serve to keep the wings together in flight.
As in Volvariella, the stress which drives the straightening of the convoluted gills of Coprinopsis is a function of the expansion of the maturing primordium. A crucial aspect of understanding how the final structure of the fruit body is attained is appreciation of the geometrical consequences of the differential growth of the primordium. For example, as a typical fruit body of C. cinerea grows from 1 to 34 mm in height, the circumference of the stem increases 9-fold and the circumference of the outer surface of the cap increases 15-fold; this latter corresponds to more than a 3000-fold increase in volume. The differential growth which generates primordium enlargement exerts enormous mechanical effects on relationships between tissue layers which are often concentrically arranged. Mechanical forces themselves generate many of the patterns which characterise the form and structure of the mature mushroom fruit body.
Temporal sequencing: hymenium assembly in Coprinopsis. Construction of the Coprinopsis hymenium seems to depend on a series of events occurring in a particular temporal sequence. First is the establishment of a cell layer comprised of basidia with a scattering of cystidia, formed by a synchronised cessation of apical growth by the probasidia (the majority cell type). The distribution of cystidia is determined by each exercising control over a morphogenetic field extending over a radius of about 35 μm which inhibits emergence of a neighbouring cystidium (explained below [view now]). Cessation of apical growth by the apical (probasidial) hyphal compartment seems to remove a constraint on branching and lateral proliferation of the sub-basidial cells. The branches which then arise from the sub-basidial compartments insert themselves into the hymenium as paraphyses .
One can accordingly write a sequential list of major controls which have to be exercised to construct the hymenium:
- specification and determination of the apical differentiation of tramal hyphal branches into basidia;
- specification of cystidial distribution;
- adhesion of cystidia spanning the gill space to cystesia in the opposing hymenium;
- as a consequence of basidial differentiation (including arrestment of extension growth of the basidial hyphal apex), emergence of paraphyses as sub-basidial branches which insert between the basidia before arresting apical wall growth and initiating the spherical wall growth which then inflates the paraphyses so that they become the main structural component as a pavement from which basidia and cystidia protrude (Rosin & Moore, 1985a; Rosin & Moore, 1985b; Rosin, Horner & Moore, 1985; Horner & Moore, 1987).
Pattern formation and morphogenetic fields. The dearth of prior research on experimental fungal developmental biology forced me to seek parallels between fungi and other eukaryotes, to make use of the conceptual framework which has been established in embryology and cell and evolutionary biology. What follows in the next few paragraphs derives mainly from the classic researches on animal embryology of the early twentieth century. Within developing tissues, cells embark on particular routes of differentiation in response to the playing out of their intrinsic genetic programme, in response to external physical signals (light, temperature, gravity, humidity), and/or in response to chemical signals from other regions of the developing structure. These chemicals may be termed organisers, inducers or morphogens, and seem to inhibit or stimulate entry to particular states of determination. Chemical signals may contribute to a morphogenetic field around a structure (cell or organ) which permits continued development of that structure but inhibits formation of another structure of the same type within the field (Moore, 1998a; pp. 1-4 and chapter 6, section 6.1.9, p. 281 et seq.).
Key words at each stage of development in fungi are: competence, induction and change. Developmental change occurs when the competent tissue is induced to progress its development. Competence can be a matter of being in control of sufficient nutrients and their translocation to particular hyphae. Induction may depend on environmental variables like illumination and temperature; and surface topology and chemicals on the surface of the host plant can trigger germination of pathogenic fungal spores and induce differentiation of germ tubes into appressoria (infection structures). Even the cessation of active mycelial growth may induce a differentiation pattern. Reproductive structures frequently arise on solid medium when growth had been checked, either by physical or chemical means. This is known as the edge effect because it is frequently expressed as a localisation of fruit bodies around the edge of Petri dish cultures which have been filled by the fungal mycelium. It is, though, the result of a correlation between an arrest to hyphal elongation and the induction of fruit bodies.
However the word 'induction' has taken on a particular meaning in fungal developmental biology as a result of the extensive anatomical studies of Bert Reijnders (Reijnders, 1977; 1993). In most fungal tissues there is a repetitive substructure comprised of a central hypha (which may remain hyphal) and an immediately-surrounding family of hyphae which differentiate in concert. These hyphal aggregations were identified and termed hyphal knots by Reijnders (1977, 1993). The indications are that the central hypha induces the differentiation of its surrounding family. If this is the case, then any control exerted by morphogens must be imposed on the central induction hypha which may not differentiate itself, but simply relay the message to its dependent family. This two stage process may influence the physical characteristics of the morphogen(s), but it might also influence their number. If the induction hyphae determine the terminal differentiation of their surrounding family, the morphogen(s) may simply need to instruct a differentiation process to occur, without defining the nature of that differentiation. The latter might be determined by local environmental, physical or nutritional factors.
The processes of competence, induction and consequential change in differentiation state all contribute to the pattern formation which characterises the ‘body plan’ which is created by the particular distribution of differentiated tissues in the structure (organ or individual) being formed. Pattern formation depends on positional information, which prompts or allows the cell to differentiate in a way appropriate to its position in the structure. Positional information is usually thought to be conveyed by concentration gradients of one or more morphogens emitted from one or more spatially distinct organisers. The responding cell senses the concentration of the morphogen and initiates a differentiation programme appropriate to the physical position at which that morphogen concentration is normally found. In essence, the cell ‘triangulates’ on the incoming signals and adjusts its morphogenetic response in accord with its position relative to the controlling organisers. Populations of cells which respond like this are said to show regional specification. The operation can be divided into (i) an instructive process which provides positional information, and (ii) an interpretive process in which the receiving cell or tissue responds and reacts to that information (Moore, 1997; Moore, 1998a; Moore, 1998b; Moore, 1998c).
The basic rules of pattern formation seem to be that regional specification (directed by organisers producing morphogens) occurs first, regulating gene activity in ways specifically geared to morphogenesis so that particular cells are first specified or committed (two equivalent names for a state which is still flexible) and then determined (a state which is inflexible or irreversible) to their differentiated fates. Cell differentiation is a consequence of these events and is the particular fate to which the cell has been specified (committed) or determined. If a cell is in a determined state, the cell’s fate cannot be reversed or transformed. Cells which are either specified or determined are not necessarily morphologically different from their neighbours or predecessors (the morphological change may occur much later, or the differentiation may not be a morphological alteration, but involve only change in molecular or metabolic attributes). This vocabulary highlights the major events contributing to animal development (embryology) and is useful, too, in descriptions of plant morphogenesis. Fungal developmental biology has been rather ignored as a topic in its own right; the great majority of the published research on fungal morphogenesis has been done with taxonomic intentions. It has great value for its descriptive and comparative content, but precise developmental accounts are extremely rare and experimental approaches rarer still (Watling & Moore, 1994).
Fungi have attributes that are unique to them which must affect their developmental mechanisms. Fungi are ‘modular organisms’, like clonal corals and vegetatively-propagated plants, among others. In modular organisms growth is repetitive and a single individual (though the definition of ‘individual’ is open to debate) will have localised regions at very different stages of development. The constituent cells are generally considered to be totipotent (able to follow any pathway of differentiation), because a mycologist would expect to be able to produce a tissue (mycelial) culture in a culture dish from a fragment removed from a mature, fully differentiated structure, like a mushroom fruit body, collected from the field. This cannot be done routinely with animals, and most plants demand far more stringent in vitro growth media and conditions than do most fungi. This behaviour reflects the nutrient-absorptive fungal lifestyle, but it also says something about the control of fungal development because even highly differentiated fungal cells will revert readily to vegetative growth if they are explanted to a (relatively simple) nutrient medium (Moore, 1998a; see In vitro tissue transplantations page [view now]). This is not to say that fungal cell differentiation is any less sophisticated or complex than is found in animals and plants, but fungi can vary the timing, extent, and mode of differentiation in response to external signals, interconverting growth forms and reproductive phases of their life cycle in ways which make them supremely adaptable to challenging conditions. This results in a morphological plasticity which surpasses that of other organisms and provides an intellectual challenge in terms of developmental biology, taxonomy, evolution and genetics (Moore, 1993; Watling & Moore, 1994; Moore, 1998b; Moore, 1998d; see discussion of fuzzy logic in the section Molecular genetic aspects of fungal developmental biology [view now]).
Prime examples of pattern forming growth factors are retinoic acid (which regulates the position of digits in the limb bud) in animals and plant auxins (which establish polarised differentiation of vessels and cell shape during vascular differentiation in plant embryos). No fungal parallels of these examples are known with certainty. Yet it is abundantly clear that cell and tissue patterns are established during development of fungal multicellular structures. Many of these patterns are akin to others found in animals and plants and, like them, tissue patterns in fungal fruit bodies are probably best interpreted as arising through the activity of ‘morphogens’ acting at a distance (Moore, 1993; Moore, 1984b; Reijnders & Moore, 1985), which may therefore be viewed as short-distance growth hormones (Novak Frazer, 1996). A variety of chemicals and extracts have the ability to induce or enhance fruiting in higher fungi and application of even simple nutrients and other metabolites can induce fruiting in some species. At intervals over many years there have been claims of experimental evidence for growth hormones in mushroom fungi. Most of these reports lack numerical data and adequate controls. Failure to establish any consistent mechanistic model from even the best documented of these accounts is very disappointing, especially in comparison with the success achieved in the parallel (and contemporary) search for, isolation, characterisation and commercial exploitation of plant growth hormones, particularly the auxins. Many of the experimental approaches used with mushrooms echo those used in the plant work and have relied on the observation that partial removal of the cap often results in curvature of the stem with the greatest amount of stem growth occurring under the remaining cap. Consequently, the one consistent conclusion which has been drawn from all of this work is that growth of the mushroom stem depends upon the continued presence of the cap. This has always been taken to imply that cap tissues produce growth hormone(s). However, as is shown above, this is not true for Coprinopsis in which stem growth and gravitropic curvature take place normally when the cap is removed. Much of the literature has been reviewed by Gruen (1982) and Novak Frazer (1996), and I recommend the discussion in Moore (1998a; see section 6.1.7, pp. 268 et seq.).
Major tissue domains in the mushroom fruit body (cap, stem, basal bulb, etc.) are established very early in the development of the structure. Some observations provide evidence for control of patterning by diffusion of chemical morphogens in morphogenetic fields around differentiating cells. As discussed below, even the generation of the agaric gill can be understood in terms of organising centres which interact with one another by production of (and response to) freely diffusing activator and inhibitor molecules (Moore, 1993; Moore, 1998b).
Knowledge of the seat and direction of the growth which drives morphogenesis and appreciation of the balance between physical and biological phenomena are essential features in understanding how tissue patterns are established. Our research on these aspects has used a range of experimental approaches (Bourne, Chiu & Moore, 1996; Chiu & Moore, 1996), and I show elsewhere in this discussion how a number of transplantation and bioassay experiments have been used to identify the sequence of commitment steps culminating in the final (determined) differentiated stage of sporulation in the meiocyte (basidium) [view now]. Other studies have identified the independent but co-ordinated morphogenetic subroutines which form a normal fruit body [view now] and demonstrated the probability that morphogens are in control of the fruit body tissue patterning.
|
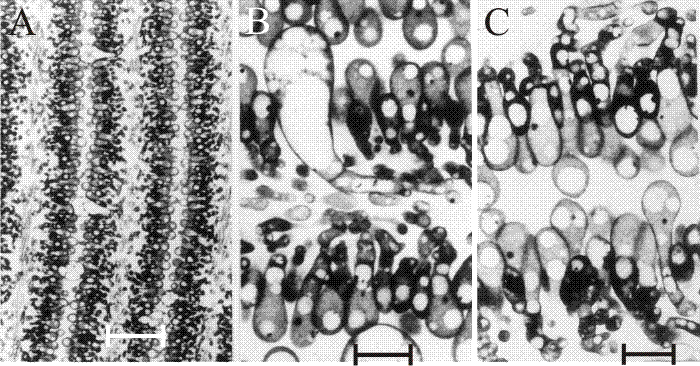
Fig. 31. The differentiating hymenium of Coprinopsis cinerea in light micrographs of sectioned tissue. A (scale bar: 20 µm), B and C (scale bars: 5 µm) show the hymenium of a primordium 1 to 2 mm tall. They reveal that at this stage the hymenium consists of a closely packed layer of young basidia, with occasional large, but still young, cystidia. The branches from sub-basidial cells that become paraphyses are evident as more densely-stained hyphal tips, beginning to force their way into the basidial layer. Photomicrographs by Isabelle V. Rosin; illustration modified from Moore (1998a). |
Rosin & Moore (1985a) demonstrated that in Coprinopsis cinerea the gills differentiated from the undifferentiated ground tissue (protenchyma) before formation of an annular cavity in a region corresponding to the boundary between stem and cap tissues. A particular pattern of branching gives rise to vertical ridges of small, closely packed cells, and as this wave of differentiation progresses towards the outer surface of the cap it leaves behind two organised plates of columnar cells (that are apical cells of hyphal branches) which constitute the primordial hymenia of adjacent gills separated by a developing gill cavity (Figs 32 and 33). The annular cavity arises only after gills are well formed and arises as a result of the radial expansion of the cap pulling the hymenial plates apart (see also Chiu & Moore (2000) and the discussion of programmed cell death in the next section [view now]). Tramal tissues of primary gills remain intimately connected to the periphery of the stem for a considerable time, becoming secondarily freed only in well-developed fruit body primordia. The morphogenetic polarities established during differentiation are maintained throughout fruit body development. From the earliest stage right through to maturation and autolysis, developmental changes proceed along two major vectors: from inner edge of the gill, i.e. the edge closest to the stem, towards the outer edge (that closest to the pileipellis - the cap ‘epidermis’); and from the cap (bottom) margin towards the cap apex. The implication is that there are organising centres for the gill plates (gill organisers) within the protenchymal tissue that progress along these vectors leaving the differentiated gills in their wake. The spatial arrangement of the gills emerges from interactions between these organising centres. Two neighbouring centres are able to inhibit the formation of a third between them until their radial growth into the expanding fruit body separates them by a distance which releases undifferentiated tissue from the inhibitory influence emanating from the existing organising centres. There is, in other words, a morphogenetic field around the gill organising centre within which new centres cannot form (Rosin & Moore, 1985a; Moore, 1987).
|
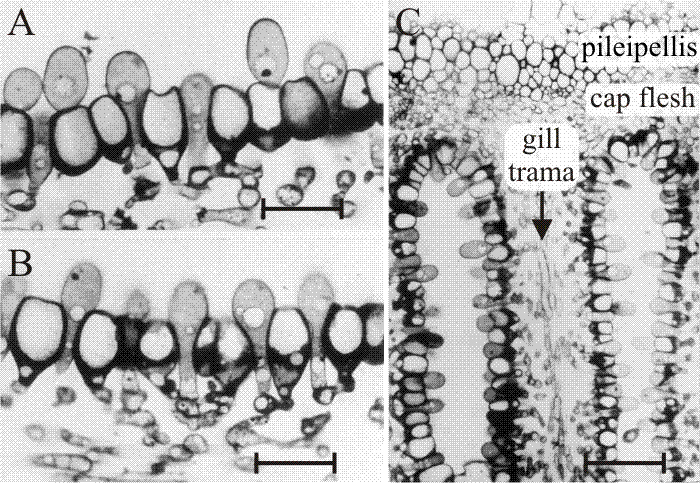
Fig. 32. The differentiating hymenium of Coprinopsis cinerea in light micrographs of sectioned tissue.Images A, B(scale bars: 10 µm) and C show views of sections of a later stage in which paraphysis insertion into the hymenium has been accomplished and paraphyses have begun to inflate. Their connection to sub-basidial cells is still very clear. C shows that the pileipellis (‘epidermis’ of the cap) is a layer made up of closely packed cells, like the hymenium. However, the cap flesh is a very open tissue with large intercellular spaces, and the same is true of the gill trama (the gill flesh) in which the hyphal nature of the tissue is clear. Photomicrographs by Isabelle V. Rosin; illustration modified from Moore (1998a). |
These studies also established the points that (a) agaric gills are initially convoluted, being stressed into their regular radial arrangement later by the mechanical forces generated by expansion of the cap (Chiu & Moore, 1990b; discussed above [view now]); and (b) agaric gills grow (that is, extend) at their base, not their margins. The spore-bearing gills of agaric fungi are plates of differentiated tissue suspended from the fruit-body cap. Intuitively, it would seem likely that such plates would develop and extend by ‘downward’ growth of the distal edge of the gill; but this is not the case.
The direction of gill development is no idle pursuit of some fine mystical point but a fundamental question of fruit-body construction. Control of morphogenesis must be exercised in the growing region; so it is necessary to know which region is growing before anything can be said about developmental regulation in the mushroom fruit body. The crucial question is whether gill differentiation proceeds radially away from the stem or radially towards the stem in the rather tubular-campanulate coprinoid primordial cap, or, irrespective of orientation, whether gills generally extend by growth at their edge or at their root (or both, or neither). The observations that establish quite categorically the direction of differentiation in Coprinopsis cinerea are as follows:
- The primordial coprinoid cap encloses the top of the stem. Since gills are formed as essentially vertical plates arranged radially around the stem, their mode of formation can be followed in transverse sections of the whole fruit body. Such a section presents the cap as an annulus surrounding, and concentric with, the stem, the inner circumference of the annulus being represented by the surface of the stem and the outer by the surface (pileipellis) of the cap tissue. These circumferences increase enormously as the fruit body grows: the former by a factor of 9 and the latter by a factor of 15 as typical fruit-body primordia grow from one to 34 mm in height. This simple fact has some decisive geometrical consequences.
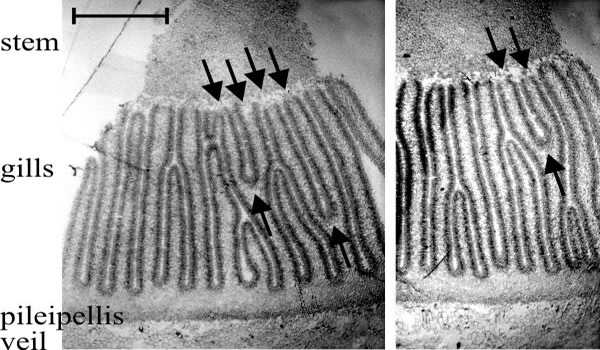
- There are two types of gill in even the youngest primordia: primary gills which, from formation, have their tramal tissue in continuity with the outer layers of the stem, and secondary (and lesser ranked) gills in which the hymenium is continuous over the gill-edge. Considering first the primary gills, one would expect their intimate tramal connection with the stem interiorly and cap tissue exteriorly to be prone to widening as both cap and stem circumferences (topologically, the surfaces to which the hymenia of primary gills are anchored) increase during maturation. But since mature Coprinopsis gills are narrow this cannot proceed to the full extent of the circumferential increase, so such a model would require that the widening be compensated by gill replication, that is, formation of a new gill cavity and its bounding pair of hymenia within the trama of a previously formed primary gill. This would result in a Y-shaped ‘replication fork’ pointing in the direction of morphogenetic development of the new hymenia. Of course, an alternative model would be that increase in the circumference of cap and stem is accommodated by growth between the preformed gills, to open-up space in which secondary gills might arise; but in this case no Y-shaped figures would be seen in transverse sections of the Coprinopsis cap. Such figures are seen, however (Fig. 33), and they are always oriented with the replication fork pointing away from the stem. Given both the bifurcation of the gill and the trama-stem connection of both parts, there is no geometrically satisfactory alternative to the conclusion that primary gills extend morphogenetically away from the stem; their roots extending into the cap tissue as the undifferentiated protenchyma is continually renewed in the outer layers of cap flesh (Rosin & Moore, 1985a; Rosin & Moore, 1985b; Moore, 1987).
|
Fig. 33. Two transverse sections of a fruit body cap of Coprinopsis cinerea. Stem tissue is at the top. All of the gills which have their tramal tissue continuous with the stem are primary gills. Note that two of these in one section and one in the other show a Y-shaped profile, in which both arms of the Y are still connected to the stem (arrows). This indicates that primary gills proliferate by formation of a new gill space within the trama of a pre-existing primary gill. Light micrographs by Isabelle V. Rosin. Illustration modified from Moore (1998a). |
We therefore conclude that the roots of the gills extend into the steadily replenished undifferentiated tissue of the cap flesh under the formative influence of the gill organisers located in the tissue at the extreme end of the gill cavity; and that the thickness and pattern of gills in any particular fruit body is the result of interactions between the morphogenetic fields of those gill organisers.
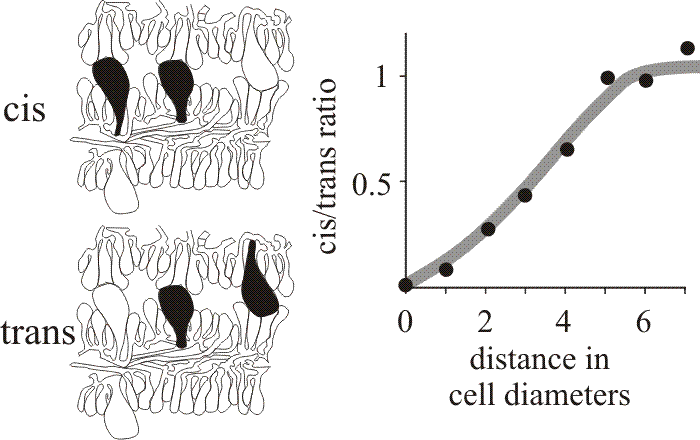
When first formed the hymenium of Coprinopsis cinerea consists of probasidia and a scattering of cystidia. The latter arise as the terminal compartments of little-branched tramal hyphae. Paraphyses originate as branches of sub-basidial cells and insert into the basidial layer. About 75% of the paraphyseal population inserted as meiosis was completed, the rest at later stages of development. Basidial numbers did not increase once paraphyseal insertion commenced (Rosin & Moore, 1985b). Another example of a morphogenetic field was recognised by analysis of the distribution of the cystidia in the hymenia of C. cinerea which was shown to be statistically non-random (Horner & Moore, 1987). Cystidia are large, inflated cells which are readily seen in microscope sections so their relationships are open to numerical analysis. Horner & Moore (1987) examined sections of C. cinerea gills prepared for the light microscope and categorised cystidia spanning the gill cavity as ‘distant’, having other cells separating them, or ‘adjacent’, with no intervening cells. In either case, neighbouring pairs of cystidia which emerged from the same hymenium were described as cis, or as trans when they emerged from opposite hymenia (Fig. 34). We argued that if the distribution of cystidia was entirely randomised there should be an equal number of cis and trans in both the distant and adjacent categories. However, quantitative data showed a distinct shortage of adjacent cis cystidia (Fig. 34), suggesting that formation of a cystidium lowers the probability of another being formed in the immediate vicinity (Horner & Moore, 1987). The plot shown in Fig. 34 suggests that inhibition extends over a radius of about 30 µm and is limited to the hymenium of origin. On the basis of these observations, the region around a cystidium was identified as a morphogenetic field controlled by the cystidium at its centre.
|
Fig. 34. Cystidium distribution in the Coprinopsis cinerea hymenium. Drawings at left show the categorisation of neighbouring pairs of cystidia in micrographs as either cis (both emerge from the same hymenium) or trans (emerging from opposite hymenia). The graph compares the frequencies of these two types over various distances of separation and shows that closely-spaced cis neighbours are less frequent than closely-spaced trans neighbours, implying some inhibitory influence over the patterning of cystidia emerging from the same hymenium. |
Cystidial density distribution on the face of the gill is fairly uniform, but at the gill edge the density of cystidia is locally increased. We suggested that differentiation leading to cystidium formation is activated by the concentration of a component of the atmosphere, possibly water vapour, in the gill cavity immediately above the developing hymenium. The distribution pattern of cystidia is thus dependent on interplay between activating and inhibiting factors. At early stages in growth of the cystidium across the gill cavity the cell(s) with which the cystidium will come into contact in the opposing hymenium are indistinguishable from their fellow probasidia. However, when the cystidium comes firmly into contact with the opposing hymenium, the hymenial cells with which it collides develop a distinct granular and vacuolated cytoplasm, more akin to that of the cystidium itself than to the neighbouring probasidia. This suggests that in this case a contact stimulus sets in train an alternative pathway of differentiation leading to an adhesive cell type which we called the cystesium (Horner & Moore, 1987).
Implicit in these interpretations is that the distribution pattern of cystidia is dependent on interplay between activating and inhibiting factors. In this instance (as with the determination of gill patterning mentioned immediately above) the patterning process is open to interpretation using the activator-inhibitor model developed by Meinhardt & Gierer (1974; Meinhardt, 1984) which supposes that morphogenetic pattern results from interaction between an activator which autocatalyses its own synthesis, and an inhibitor which inhibits synthesis of the activator. Both diffuse from the region where they are synthesised, the inhibitor diffusing more rapidly and consequently preventing activator production in the surrounding cells. This results in long-range inhibition and short-range activation. Increase in the activator is eventually arrested (limited, for example, by diffusion) and stable activator and inhibitor concentration profiles are established. A wide variety of patterns can be generated in computer simulations by varying diffusion coefficients, decay rates and other parameters (Meinhardt, 1984). The model readily accounts for stomatal, cilial, hair and bristle distributions, and has been applied successfully to simulate leaf venation and phylotaxy (Meinhardt, 1984); there’s no reason why the model should not apply to the distribution of fungal structures. And that conclusion seems to establish the point that the mechanistic logic of morphogenesis transcends all three eukaryote kingdoms even though they independently evolved their mechanisms to control their multicellularity. [TOP]
Fungal Morphogenesis Fungal Morphogenesis is Volume 35 in the prestigious Cambridge University Press series on Developmental and Cell Biology, joining books on topics as diverse as kidney organogenesis and the development of fern gametophytes. Moore’s intention is stated in the Preface: “I believe the fungi are too important to remain in an intellectual ghetto in some faintly plant‑like place which most people visit rarely, and then with unease.” He wants the scientific world to appreciate the unique attributes of fungi and at several points emphasises that fungi are not plants. Animals engulf food. Plants manufacture food. Fungi are absorptive. The life style differences between animals, plants, and fungi are crucial to determining their characteristic shapes and forms. Fungal morphogenesis is addressed as a direct manifestation of biological diversity (i.e. fungal development as a topic in its own right) and within the context of broader biological significance (i.e., fungal development as a model for understanding molecular control of morphogenetic events). … Experimental observations, in which specific cases are studied under particular conditions, must be weighed and synthesised in order to make generalisations. Moore admits “It is necessary to take the risk of being wrong in order to get some view of the overall picture,” and does an admirable job of making provocative but not reckless hypotheses that can be tested in the laboratory. One particularly appealing aspect of the book is the author’s familiarity with, and ready usage of, the zoological literature. Fungal morphogenesis is placed within the wider literature on animal pattern formation. Much can be learned from the embryology literature. Moore's erudition also informs his coverage of secondary metabolism. It is often stated that bacteria, fungi and plants are the main producers of these natural products. Moore points out that the supposed deficit of metabolite production by animals may be a matter of semantics. Animals produce toxins, hormones and other low molecular weight metabolites that can be viewed as secondary metabolites labelled with a different name. ... my opinion is highly positive. Moore is well read. He writes well and thinks broadly. He has the rare ability to describe both the forest and the trees. Since morphogenesis is the study of the whole process by which the organisation and pattern of an organism is established, a book on fungal morphogenesis must integrate biochemical, structural, genetic and molecular mycological research. Moore’s synthesis is a tour de force. It is rare to find a single author work that covers such a wide breadth of topics with such a pleasing combination of knowledge and eloquence. Tucked between the title page and the table of contents, Moore prints a John Lennon lyric: “Living is easy with eyes closed! Misunderstanding all you see” (Strawberry Fields, 1966). Mycologists and other biologists who delve into Fungal Morphogenesis will come away with a restless scientific unease over the extent of our ignorance and misunderstanding. This is Moore’s intent. The final chapter of this fine monograph includes an apology “This will involve extensive generalisations and potentially outrageous extrapolations” and a challenge: “Do the research to prove me wrong.” All developmental mycologists should own a desk copy of Fungal Morphogenesis and all science librarians should be alerted to order a copy for their collections. Joan Bennett, Department of Cell & Molecular Biology, Tulane University, New Orleans; from Inoculum: Supplement to the journal Mycologia Vol. 52(1) February 2001, and Newsletter of the Mycological Society of America. |
| A review of Moore, D. (1998a). Fungal Morphogenesis. Cambridge University Press: New York. ISBN-13: 9780521552950. DOI: http://dx.doi.org/10.1017/CBO9780511529887. |
[TOP]
Updated December 7, 2016