8. Programmed cell death
Morphogenesis can require the removal of tissue as well as tissue growth and the cell death responsible for this must be controlled in time and position. This is programmed cell death; it is an important aspect of development in plants and animals. Recent observations have shown that in fungi, too, hyphal compartments are sacrificed to trim hyphae to shape and tissues are sculptured by extensive sacrifice of hyphae.
If the death of cells can be regulated and, more importantly in a hyphal system, if the death of hyphal compartments can be strictly localised, then cell death could be used as a morphogenetic process. Such a mechanism occurs in animal development and there is probably a need for a similar mechanism in fungal morphogenesis. In animals there are two patterns among dying cells (Sen, 1992). Traumatic or necrotic death occurs when the cell is suddenly confronted with extreme non-physiological conditions and loses control of its ionic balance. As a result calcium enters the mitochondria (causing swelling and dilation) and the cytoplasm becomes hypertonic. Uncontrolled water influx causes the cell and its organelles to swell and lyse.
On the other hand, apoptotic or programmed cell death occurs in physiological conditions, often in response to effectors which are not lethal to other cells in the vicinity. Indeed, some mammalian cells seem to be programmed for apoptotic suicide unless suppressed by signals from other cells. Apoptotic death is relatively slower than necrosis and involves a programme of well regulated processes (including synthetic ones) which lead to internal cell degeneration and eventual removal of the dying cell by phagocytosis. Apoptosis is of enormous importance in organ development during embryogenesis where cell elimination is a key feature in morphogenesis (though only a very small minority of cells, less than 1%, may be undergoing the process at any one time).
Apoptotic cell death is also important in higher animals as a mechanism whereby autoimmunity can be avoided or minimised. Necrotic cell death releases all of the cell machinery to become potential antigens. In apoptotic death the components of the dying cell are digested within its membrane prior to phagocytosis so in most cases no antigens escape. Obviously, this last point is not a consideration in fungi. But it is particularly interesting and significant that Umar & Van Griensven (1997a) identified two modes of cell death in A. bisporus fruit bodies; one impacting as a trauma from the outside which can be likened to necrotic death, and the other which is more like a true, internally driven, senescence. This latter can be considered a fungal type of programmed cell death.
Moore (2003) pointed out that although the Oxford Dictionary of Biochemistry and Molecular Biology (Smith et al. 1997) defines apoptosis as ‘cell death; used broadly to encompass all forms of normal or pathological cell death or may be confined to those processes involving morphological changes such as occur in normal animal development’ the lack of mention of fungi or plants simply reflects that regrettably frequent narrowness of mind that leaves so many people thinking that only animals matter. There’s no doubt at all that plants use programmed cell death (think of all the leaves falling from those deciduous trees in autumn) and as you might expect, fungi are well versed in the sophisticated use of programmed cell death (PCD and have been using it for a long time. Some recent papers establish this point, but we will find evidence for it in some of the classic literature, too. Mousavi & Robson (2003), for example, have demonstrated that cell death of Aspergillus fumigatus in the stationary phase depends on caspase-like enzyme activity. This is similar to animal apoptosis, where cysteine-proteinases known as caspases are essential components of the apoptotic pathway.
Similarly, Lu, Gallo & Kües (2003) present customarily elegant cytological evidence for apoptotic DNA degradation in basidia of meiotic mutants of Coprinopsis cinerea (as Coprinus cinereus). Again, there is a compelling comparison with animal apoptosis where specific DNA fragmentation is an essential component. As Money (2003) has commented, though, such a process in a mushroom may well be a matter of resource conservation (the Coprinopsis mutants cannot complete meiosis so there is presumably some value in recycling the contents of the defective basidia). However, constant comparison with apoptosis in complex animals may well be diverting us from appreciation of the several roles for programmed cell death in fungi that have been known for many years. The point is that most research is done (for obvious reasons) on vertebrate animals in which a primary ‘design requirement ’ is that as the cell dies release of antigens must be avoided to protect the animal against autoimmunity. This has resulted in development of a highly sophisticated cell destruction process that takes place inside an intact cell membrane, so that the cell remnants remain separated from the immune system until they are engulfed by phagocytes. This seems to be the system with which everything else is compared, but it is a highly adapted system, and even in animals there are many examples of ‘partial apoptotic ’ systems which, nevertheless, accomplish programmed removal of cells in a controlled and specific manner (Lockshin, Zakeri & Tilly, 1998). So, the message is that a cell death programme will be adapted to its function, and, unless it happens in an organism with an active immune system, don’t expect it to feature all the events that can be recognised in vertebrates.
Moore (2003) also drew attention to the claim that the phrase ‘programmed cell death’ was first used in a doctoral thesis dealing with insect development (Lockshin, 1963). Although more than 30 years earlier Buller (1924, 1931) interpreted autolysis in coprinoid fungi as part of the developmental programme of the fruit body (specifically: autolysis removes gill tissue from the bottom of the cap to avoid interference with spore discharge from regions above). I consider that Reginald Buller deserves a healthy slice of any credit that might be handed out for introducing this concept into biology.
Umar & Van Griensven (1997a) found that the life span of fruit bodies of A. bisporus was 36 days when grown in a cultivation environment which protected the culture from pests and diseases. Senescence first became evident around day 18, with cytological indications of localised nuclear and cytoplasmic lysis. These changes were followed by increased permeability of the cytoplasmic membranes and by structural changes to the cell wall. Remains of the lysed cells aggregated around and between the remaining hyphal cells. Most of the stem hyphae became empty cylinders. Other cells within the fruit body collapsed irregularly. Electron microscopy showed that most of the cells throughout the fruit body were severely degenerated and malformed after 36 days, yet a number of basidia and subhymenial cells cytologically remained alive even on day 36. When mushrooms were cultivated using conventional mushroom farming procedures, about 50% of the fruit bodies were found to have been infected by Trichoderma harzianum and/or Pseudomonas tolaasii by day 18. All such fruit bodies died on day 24 due to generalised severe bacterial and fungal infections leading to tissue necrosis and decay of the caps and stems.
In harvested A. bisporus fruit bodies (stored under various conditions) diffuse cell wall damage was observed first, this only later being accompanied by cytoplasmic degeneration. Consequently, Umar & Van Griensven (1997a) emphasise that the morphological changes which occur in naturally-senescent and post-harvest fruit bodies of A. bisporus are different. Post-harvest physiology and morphology of mushrooms is of paramount importance for mushroom marketing and has been extensively studied, but post-harvest behaviour is usually described as senescence or as an ageing process. It is quite clear from the detailed analysis of Umar & Van Griensven (1997a) that this is not the case. The harvested mushroom has suffered a traumatic injury and its post-harvest behaviour stems from that.
A major factor must be that it has no way of replacing water lost by evaporation. Consequently, exposed surfaces become desiccated and are damaged first. Thus, in what might be called 'post-harvest stress disorder', further damage is inflicted on the cell inwards, from the outside. In complete contrast, during the senescence which accompanies normal ageing, the damage is inflicted first on the genetic architecture (this to include nuclear and organelle genomes) and subsequently on cytoplasmic integrity, so that cell wall damage occurs as an aspect of the eventual necrosis suffered by the lysing cell. That is, in senescence the damage starts inside the cell and proceeds outwards, from the inside.
The most obvious example of programmed cell death is the autolysis which occurs in the later stages of development of fruit bodies of many species of Coprinus. Buller (1924, 1931) described these in detail and, as stated above, interpreted the autolysis to be part of the developmental programme. That is, when the hymenium has discharged its basidiospores, autolysis is initiated to destroy that part of the gill so that it will not be a physical barrier to discharge of spores by the remaining part of the gill. The enzymology of this gross autolysis has been examined in detail in Coprinopsis cinerea and found to be due to the release of chitinase, acid and alkaline proteinase, RNA-ase, phosphatase and β-glucosidase enzymes which had previously been localised in intracellular vacuoles (Iten, 1970; Iten & Matile, 1970). Vacuoles containing acid and alkaline proteinase, RNA-ase, phosphatase and β-glucosidase were found in vegetative mycelium as well as fruit body gill tissues, so they appear to be part of the normal turnover-metabolism of the cells. Vacuoles with chitinolytic activity were, however, newly formed shortly before spore release was initiated. Iten & Matile (1970) suggested the chitinolytic enzymes were passively released by cells whose metabolic activity had ceased; their growth thereby becoming unbalanced.
Taken together these observations show that autolytic modification of the fruit body in later stages of its development in Coprinopsis involves specific production of new enzymes in particular cells at a particular time. The autolytic destruction of those cells is clearly part of the morphogenesis of the fruit body; it is a programmed cell death. The process cannot be dismissed as simply the final step of some other developmental programme. Buller (1924) also described a much more localised autolysis of cap flesh immediately above the gill trama in small species of 'Coprinus' which enabled the gills to split and their two hymenia to be stretched apart as the cap opened like an umbrella (as in the species now called Parasola plicatilis; see http://www.mushroomexpert.com/parasola_plicatilis.html). In this case the same autolytic programme is being exercised at an earlier stage in development and is strictly limited to cells which are specifically placed to achieve a particular fruit body morphology. Indeed, this umbrella-like expansion of the cap as each gill is split and opened out to form a V-shape in cross section also applies in Coprinopsis cinerea and Muraguchi, Fujita, Kishibe, Konno, Ueda, Nakahori, Yanagi & Kamada (2008) have shown that this developmental change depends on a specific gene, exp1 (short for expansionless-1), which encodes a protein with two HMG domains. HMG stands for 'high mobility group' and refers to a class of proteins that contains one or more domains in which the amino acids form an irregular array of three helices. These domains are involved in binding to DNA, and may be involved in protein-protein interactions as well, that regulate DNA-dependent processes (including transcription) that require the bending and unwinding of chromatin. Muraguchi et al. (2008) found transcription of exp1 to be strongly induced in the cap 3 hours before normal cap expansion and they suggest that the Exp1 gene product is a transcriptional regulator controlling highly localised cell lysis (= programmed cell death) events that contribute to the final phase of fruit body morphogenesis.
Umar & Van Griensven (1997b, 1998) have indicated that there may be a more general involvement of a fungal type of programmed cell death in fruit bodies of higher fungi. It is shown that in very early primordia of Agaricus bisporus the first gill spaces are formed as a result of cell death. The authors point out that the exact timing (prior to basidial differentiation) and exact positioning (in an annulus close to the junction of cap and stem) implies that cell death is genetically programmed as part of the morphogenetic process. It would seem that fungal programmed cell death plays a role at many stages in development of many species (Umar & Van Griensven, 1998).
A need for such a mechanism is evident from the morphology of fruit body initials (Fig. 35, below). These appear to be tangled masses of long hyphae, yet very early in fruit body development compact structures emerge with clearly demarcated surfaces. In the initials, hyphae extend in every direction so demarcated surfaces can only arise if pre-existing hyphae which cross the boundary before it is established can be severed.
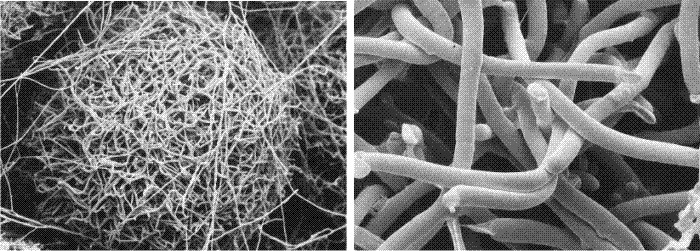 |
| Fig. 35. Cryo-SEM images of the outer layers of fruit body initials of Pleurotus pulmonarius. Many of the hyphae were alive when flash-frozen and remained fully inflated. Cells undergoing programmed cell death, however, tend to collapse. In the right-hand image a number of the hyphae show abrupt terminations where the hyphal filament has been severed by programmed death of one or more hyphal compartments. Photographs kindly provided by Dr Carmen Sánchez. |
The cytological evidence seems to indicate that individual hyphal compartments can be sacrificed (Fig. 35) in order to trim hyphae to create a particular shape. Programmed cell death is used, therefore, to sculpture the shape of the fruit body from the raw medium provided by the hyphal mass of the fruit body initial and primordium (Moore, Chiu, Umar & Sánchez, 1998).
Although, as stated above, Umar & van Griensven (1997b, 1998) demonstrated that cell death is involved in formation of gill cavities in Agaricus bisporus, no sign has been found of cell disintegration, nor evidence of cell death during gill formation in the mushroom of Coprinopsis cinerea (Chiu & Moore, 2000). Gill formation in this mushroom has been interpreted as depending on a particular branching pattern; branches of determinate growth being organised into opposing palisade cell plates, forming an incipient fracture plane. This plane is then opened out into a cavity when expansion of underlying tissue put tension across the 'fracture' and pulls the palisades apart. Morphological mutants have been used very effectively to dissect morphogenetic pathways in Coprinopsis. The work used two induced mutants of a single strain of Coprinopsis cinerea that have identical morphological abnormalities but different DNA fingerprints. The primary morphological abnormality is the failure to initiate the hymenophore subroutine, usually visible at the primordial stage. Remember that a feature of fungal morphogenesis is that it is compartmentalised into a collection of distinct developmental processes we have called 'subroutines' (see Molecular genetic aspects of fungal developmental biology, below [view now]); the hymenophore carries the hymenium, which is the cell layer responsible for eventually producing the basidiospores. The hymenophoreless mutants described by Chiu & Moore (1999d) and Chiu & Moore (2000) lack the subroutine that specifies the hymenophore, so no gills are formed. However, a cap subroutine was initiated after the stem subroutine, forming an apical 'knob' on an aerial elongated primordium. Although no differentiation of the pileipellis was evident (this being an epidermal layer that usually marks the boundary between the outermost loose and deciduous veil cells and the inner closely packed cap tissues) the stem went through the first phase of elongation and the cap did expand; so, if some form of cell disruption was necessary to generate the gill space, there was sufficient consequential mechanical stress to cause cell disruption but this was not observed. Chiu & Moore (2000) concluded that the key process in Coprinopsis is the patterning of hyphal tips into the palisades which become opposing hymenia (Rosin & Moore, 1985a), and not the formation of a primordial gill space by programmed cell death. This implies that a gill organiser (Moore, 1998b), which is responsible for patterning branches of determinate growth into opposing palisades, must be present to make gills in Coprinopsis (see Control of fungal morphogenesis section, above [view now]).
Clearly there are several ways to create gills in agarics and Agaricus bisporus and Coprinopsis cinerea employ different mechanisms. These differences may, at least in part, be a reflection of observable differences in hymenium structure between these species. The hymenium of Coprinopsis develops as a uniform layer of cells: uniform in size, structure and timing and progress of development (Rosin & Moore, 1985b). In contrast, the hymenium of Agaricus bisporus, from start to finish, is a community of cells with different functions, different rates of developmental progress and such varied functions that in many cells even meiosis is brought to a halt so that the cell can serve a temporary structural rather than an immediately reproductive function (Allen, Moore & Elliott, 1992). There’s an important unstated observation here on which Moore & Chiu (1998a) have briefly reflected: most of what is known about most aspects of mushroom biology is limited to just a few ‘text-book’ examples that are neither closely related nor sufficiently different to be representative.
The point about being related matters because as the features which are compared become more detailed, so increasing care must be exercised in making comparisons. We are now getting to the stage where the cell biological functions of many newly recognised components of the genome need to be hypothesised, but the scope of the biology on which this hypothesising can be based does not go beyond the narrow text-book description of basidiomycete biology of the mid-twentieth century. For example (in addition to the Coprinopsis/Agaricus contrast just mentioned), the enormous differences between the biology of the wood-degrading aphyllophoralean Schizophyllum commune and the litter-degrading agaric Coprinopsis cinerea seem to be in danger of escaping notice as they are swept along by an ‘if it’s got gills it must be an agaric’ school of thought. Yet, in agarics (like C. cinerea) adjacent basidia in the hymenium arise at the apex of sister branches from parental sub-hymenial hyphae. On the other hand, in aphyllophorales (like S. commune) adjacent basidia arise through proliferation at sub-basidial clamp connections. It’s not just S. commune and C. cinerea, of course, those other non-agarics Lentinula and Pleurotus are also too often discussed as though they are also just ordinary mushrooms. It might be likened to comparing two animals that swim - it’s OK if they are both fish, but one might be a dolphin. If you don’t know that dolphins exist, you could miss a lot in your comparisons! Moore & Chiu (1998a) questioned whether the ‘text-book’ basidiomycetes could adequately represent all filamentous basidiomycetes and suggested that deepening knowledge of molecular biology needs to be accompanied by deepening appreciation of the breadth of basidiomycete physiology, cell biology and field biology. This is still true in 2013 as more and more efforts are devoted to fungal genomes. [TOP]
Updated December 7, 2016










