10. Molecular genetic aspects of fungal developmental biology
It must be evident from the discussion so far that patterning genes may be difficult to find because the pattern under investigation may simply be an inevitable consequence of events which have gone before. A specific gill pattern in an agaric, say, may result because the application of general rules within the context of its development 'invariably' results in that pattern. Clearly, genetic components are essential (Figs 37 to 39) but recognising their precise phenotype may not be straightforward.
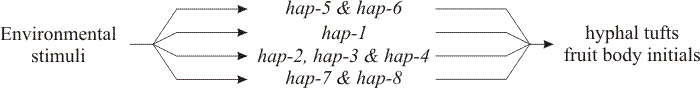 |
| Fig. 37. Genetic pathways to fruiting deduced from segregation genetics: genes involved in monokaryotic fruiting in Schizophyllum commune (after Leslie & Leonard, 1979) in which hap-5 and hap-6 control spontaneous fruit body initiation, hap-1 alone or hap-2, -3 and -4 acting together control fruiting as a response to injury, and hap-7 and hap-8 determine fruiting in response to applied chemicals. |
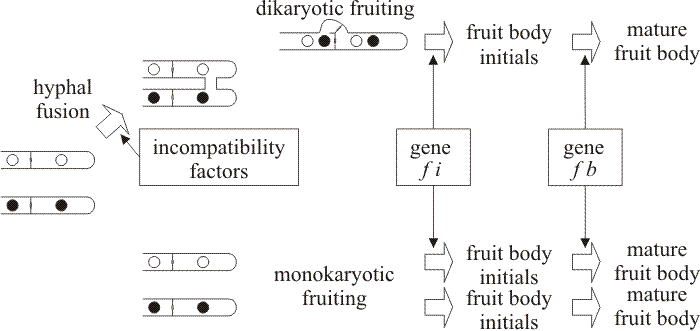 |
| Fig. 38. Genetic pathways to fruiting deduced from segregation genetics: a model for the action of major genes controlling mushroom formation in both dikaryotic and monokaryotic mycelia of Agrocybe aegerita (after Esser & Meinhardt, 1977). |
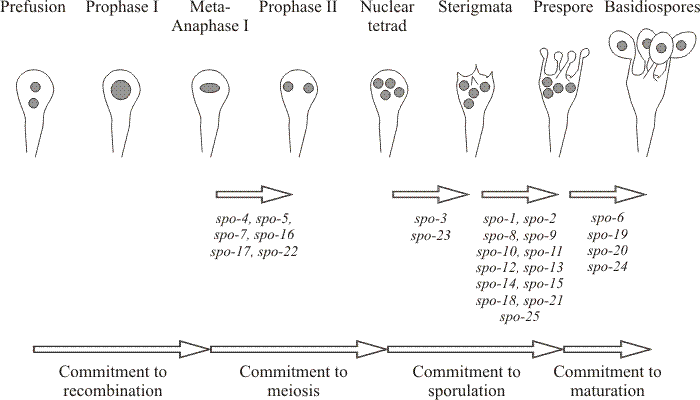 |
| Fig. 39. Genetic pathways to fruiting deduced from segregation genetics: genetical and physiological milestones in the basidial differentiation pathway of Coprinopsis cinerea. Prevention of meiosis still permits the fruit body to develop normally, demonstrating, as do monokaryotic fruit bodies (Figs 37 and 38), that meiosis and spore formation are entirely separate from construction of the spore-bearing structure. Sporeless mutants have been used to study the sporulation pathway and identify the succession of commitment stages shown here (Miyake, Takemaru & Ishikawa, 1980; Miyake, Tanaka & Ishikawa, 1980). Diagram adapted from Bourne, Chiu & Moore (1996). |
Except for the meiocyte, differentiated fungal cells have an extremely tenuous grasp on their state of differentiation. So tenuous that when removed from their normal tissue environment they revert immediately to the vegetative hyphal growth mode (see 'In vitro tissue transplantations' above).
Another common feature is that fungal morphogenesis is compartmentalised into a collection of distinct developmental processes (called 'subroutines'; Chiu, Moore & Chang, 1989; Watling & Moore, 1994; Moore, 1995; Moore, 1997). These are recognisable at all levels: including particular organs (e.g. cap, stem, veil, etc.); tissues (e.g. hymenophore, context, pileipellis, etc.); cells (e.g. basidium, paraphysis, cystidium, etc.); and cellular components (e.g. wall growth may be uniform, in girth, in length or in thickness).
Developmental subroutines are distinct genetically and physiologically and may run in parallel or in sequence. When played out in their correct arrangement, 'normal' morphology results, but if some subroutines are disabled (genetically or through physiological stress), the rest may still proceed. Such partial execution produces an abnormal morphology, but in many instances spores are still produced. This flexibility in expression of developmental subroutines allows the fruit body to react to adverse conditions and still produce a crop of spores. It also illustrates that tolerance of imprecision is an important attribute of fungal morphogenesis.
Fungal development uses fuzzy logic rather than crisp logic. Discussion of differentiation in fungi often involves use of words like 'switch' in phrases which imply wholesale diversion at some stage between alternative developmental pathways. There are now many examples, some of which have been described above, which suggest that fungal cells behave as though they assume a differentiation state even when all conditions for that state have not been met. Rather than rigidly following a prescribed sequence of steps, these fungal differentiation pathways appear to be based on application of rules which allow considerable latitude in expression. What might be thought of as 'decisions' between developmental pathways are made with a degree of uncertainty, as though they are based on probabilities rather than absolutes. For example, facial cystidia of Coprinopsis cinerea are generally binucleate, reflecting their origin as branches from the dikaryotic gill trama and the fact that they are sterile cells, yet occasional examples can be found of cystidia in which karyogamy has occurred (Chiu & Moore, 1993) or of cystidia bearing sterigmata.
Development of a cystidium represents expression of a perfectly respectable pathway of differentiation and commitment of a hyphal tip to the cystidial as opposed to the basidial pathway of differentiation. The commitment must occur very early in development of the hymenium because young cystidia are recognisable in the very earliest stages (Rosin & Moore, 1985b). The controls which determine formation of a cystidium, instead of a basidium, by a particular hyphal apex need to be established. It is certainly the case that the basidial developmental pathway (in A. bisporus) can be interrupted to allow this cell type to serve a structural rather than spore-producing function (Allen, Moore & Elliott, 1992), though this is clearly arrested meiosis, not sterility. Conceptually, this is similar to the cystidia of Coprinopsis which show evidence of entry into meiosis (Chiu & Moore, 1993) and which suggest that entry to the cystidial pathway of differentiation does not totally preclude expression of at least part of the meiocyte differentiation pathway. Similarly, the fact that a large fraction of the in situ basidial population of A. bisporus remains in arrested meiosis indicates that entry to the meiotic division pathway does not guarantee sporulation; a fact also demonstrated with excised gills of Coprinopsis cinerea in vitro (Chiu & Moore, 1990a).
Chiu & Moore (1993) discuss the possibility that fungal differentiation pathways exhibit what would be described as 'fuzzy logic' in cybernetic programming terms. The phrase has a particular meaning, which has been equated with 'computing with words' by Zadeh (1996). It is a methodology which is useful for dealing with situations which must tolerate imprecision; effectively, where the programming term 'either/or' must become 'maybe'. It is easiest to illustrate by contrasting a graph based on conventional real numbers, which describes what is called a crisp function, with a fuzzy graph (Fig. 40). Clearly, the imprecision of the input to the fuzzy graph greatly expands the fuzzy constraints beyond those defined by the crisp function. It is this notion of tolerance of imprecision which can be applied to fungal morphogenesis.
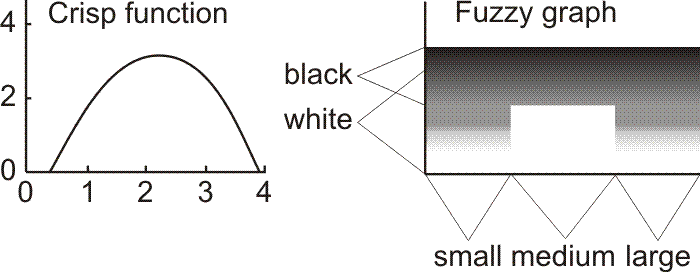 |
Fig. 40. Comparison of a crisp function (left) with a fuzzy graph (right). The peak values of the crisp function graph are specified by a precise relation between x and y, possibly with some definition of the extent of stochastic variation. On the other hand, the peak of the fuzzy graph is indicated adequately by the phrase ‘about medium size and very dark in colour’. Adapted from Zadeh, 1996. |
Instead of viewing fungal cell differentiation as involving individual major 'decisions' which switch progress between alternative developmental pathways which lead inevitably to specific combinations of features, this idea suggests that the end point in fungal differentiation depends on the balance of a number of minor 'decisions'. Observation shows that developmental decisions between pathways of differentiation are able to cope with a degree of uncertainty, allowing hyphae to assume a differentiation state even when all conditions of that state have not been met. So, rather than rigidly following a prescribed sequence of steps, fungal differentiation pathways must be based on application of rules which allow considerable latitude in expression - fuzzy constraints. This feature is expressed by abnormalities in hyphal differentiation pathways which in the ultimate can lead to polymorphic fruit bodies. The selective advantage of this system, though, is that it seems to allow even grossly abnormal tissues and fruiting structures to carry out their prime function, namely the production and distribution of progeny spores (Moore, 1998b). Fruit body structural genes are likely to function to aggregate hyphae together to form the 3-D structure of the fruit body which, in most homobasidiomycetes serves to protect the spore dispersal system from rain. Overall, the genetic structure seems to be one in which relatively few genes acting as major regulators govern entry into integrated pathways to which a large number of other genes contribute. Regulation probably occurs both at transcription and translation (Moore & Chiu, 1998b).
A fruit body carries out the functions of species propagation and, in most cases, this is coupled with the generation of genetic variation for evolutionary challenge. Genetic variation among the progeny spores of the fruit body results from the pairing of two different nuclei followed by the well-established pathway of meiotic recombination, random chromosomal segregation and migration of nuclei (see Chiu & Moore, 1999a). I started my research career establishing chromosomal linkage groups in Coprinopsis cinerea (Moore, 1967), and thirty years later contributed to a study of population diversity in cultivated and natural populations of Lentinula edodes (Chiu, Wang, Chiu, Lin & Moore, 1999). This was based on a field study carried out in a remote broadleaved Fagus longipetiolata forest in Shaanxi province, China (Fig. 21) to study the natural local distribution of Lentinula edodes. Following spatial mapping, 24 fruit bodies were collected for tissue isolation into axenic culture. Twenty-four genets distributed on fallen tree trunks within a distance of 120 m were identified and clustered into 7 groups using data based on colony morphologies, abilities to degrade aromatic dye, somatic incompatibility reaction patterns and DNA fingerprints. Among the parameters used, the somatic incompatibility reaction, a polygenic phenotype, was the most differentiating, identifying 22 incompatible classes. Two sets of fruit bodies which were different genetic individuals were so close together that they would otherwise have been described as aggregate fruits of presumed identical origin. Eighteen genetic individuals found on the same 5.6 m long tree trunk divided roughly into two clusters, matching their spatial distribution, and a nearby branch bore another distinct cluster. More heterogeneity was encountered between isolates the greater the distance separating them on the original site. Genetic individuals on the same tree trunk showed more compatible somatic reactions among themselves, and their DNA fingerprints showed higher similarity.
Nevertheless, considering the totality of phenotypic characters recorded, each fruit body is a genetic individual in L. edodes. Such features are concluded to result from a reproductive strategy which depends on basidiospore dispersal. Within each cluster of isolates from the collection site genetic individuals seemed to have arisen from multiple sib-mating events. Thus, a cluster may represent a lineage of L. edodes. Individualism in L. edodes is based on a strong somatic incompatibility system. Strong competition from contaminating individuals arriving as air-borne basidiospores could explain decreased and fluctuating crop yields of traditional mushroom farms which are now frequently observed in later flushes from the outdoor wood log cultivation system. Further, it would also explain why multispore spawn is not favoured in artificial cultivation of this economically important edible mushroom.
This analysis of Lentinula in its native habitat revealed that the organism captures only limited volumes of resource in nature because of the high level of competition it encounters, but there were no genetically-unusual findings. Yet there are still many unknowns and our related work on Volvariella volvacea is a particularly unusual example. V. volvacea was first cultivated in China in 1822 and is the most popular fresh mushroom in Hong Kong and the fifth in the world. The organism exhibits primary homothallism, but we found that fruit bodies formed on mycelia grown from haploid, uninucleate basidiospores (which should be homozygous) are nevertheless able to segregate physiological and DNA markers, and selfed Volvariella volvacea fruit bodies regularly produce a 1:1 segregation ratio of self-fertile to selfsterile progeny. All of this suggests that the organism has a mechanism (or mechanisms) to maintain variation despite inbreeding (Chiu & Moore, 1999b). Our studies found that DNA fingerprints obtained from different stages during fruit-body morphogenesis were identical. However, variation in DNA fingerprints was evident in protoplast regenerants derived from the same vegetative mycelium suggesting that the haploid mycelium formed from haploid, uninucleate basidiospores from self-fertilised fruit bodies somehow became heterokaryotic. Considering the various mechanisms known to generate genetic variation, and bearing in mind my theoretical and experimental experiences more than twenty years earlier with recombination mechanisms in Coprinopsis (Moore, 1972, Moore, 1974a, Moore, 1974b, Moore & Katy, 1978) we advanced an explanation based on the absence of repair of base mismatches in hybrid DNA generated during normal meiotic recombination in the homothallic diploid nucleus. The result of this would be that:
- in every meiosis at least a proportion of the heterozygosity of the diploid nucleus would be preserved in the haploid daughter nuclei in the form of base mismatches in hybrid DNA;
- at the first postmeiotic mitosis (in this organism this occurs at basidiospore germination) these mismatches would be segregated into daughter nuclei and the V. volvacea spore germling would immediately become dikaryotic, even though the basidiospore had been haploid and uninucleate;
- assuming that recombination and mismatch-repair patterns differed between different meioses then different spores would produce different dikaryons.
Consequently, we suggested that uncorrected hybrid DNA was the source of the variation in segregation which was observed (Chiu & Moore, 1999b). [TOP]
Updated December 7, 2016










