EctomycorrhizasCompared to arbuscular mycorrhizas (AM), the range of plants colonised by ectomycorrhizas (ECM) is relatively small. A mere 3% of phanerogams (seed plants) are ECM, all of which are woody species; trees and shrubs. Despite this small percentage, the worldwide importance of the ECM association is greatly increased by the large area covered by the plants globally, and their economic value as the source of timber. ECM symbioses are believed to be more common in the temperate zones of the world than the tropics. In the northern temperate regions, plants such as pine (Pinus), spruce (Picea), fir (Abies), poplar (Populus), willow (Salix), beech (Fagus), birch (Betula) and oak (Quercus) typify the ECM association. In the southern hemisphere Eucalyptus and Northofagus (Southern Beech) are important genera as is the Dipterocarpaceae family, found in the monsoon forests of south-east Asia. In total, 140 genera, in 43 plant families have been identified as forming ECM. In contrast to the AM association, a wide range of fungi form ECM. At least 65 genera have been identified, the majority of which (45 of 65) are Basidiomycota. Eighteen genera of Ascomycota are represented and also the Zygomycete genus Endogone, which can form AM as well. Classification has been by identification of the fruiting body in the past, and since these have not always been observed, some fungi remain unclassified. It has been estimated that between 5,000 and 6,000 species of fungi form ecto- or ectendo-mycorrhizas. Around 4,500 of these are epigeous (have above-ground fruiting bodies), but up a quarter are hypogeous (with underground fruiting bodies) such as truffles (Fig. 1). Members of the Ascomycota are particularly well represented by hypogeous forms. The ECM fungi do not show a high degree of host specificity, either in natural or artificial conditions. It is common to find mycorrhizas belonging to several different fungi on the root system of a single tree. Norway spruce (Picea albies) can form ECM symbioses with over 100 different fungal species and the fly agaric (Amanita muscaria; Fig. 2), can infect the roots of trees as varied as birch, eucalyptus, spruce and Douglas fir.
The pattern and succession of mycorrhizal development varies during the life of a tree. Some fungi are successful primary colonisers, the 'early-stage' fungi, whilst different fungi dominate as the tree ages and reaches maturity, the 'late stage' fungi. However, some fungi will infect regardless of stage, and seedlings germinating in a mature forest are usually colonised by the dominant 'late' or 'multi' stage fungi. The 'early stage' fungi seems to have more of a role where their plant partner is a pioneer colonist, and there are few other soil fungi present. The 'early stage' fungi act like ruderal or r-selected species, and those of the 'late stage' are k-selected organisms, as they are unable to survive in soil not already inhabited by other mycelia. The difference between these types of fungi is important commercially, as the 'early stage' fungi can be used as inoculants to stimulate new forest growth, whereas the 'late stage' fungi cannot. ECM infection has been shown to be necessary for the successful establishment of some trees, such as Pinus, and can allow seedlings to compete against mature trees in less favourable conditions, such as shading. The characteristic features of the ECM association are (Figs 3 & 4):
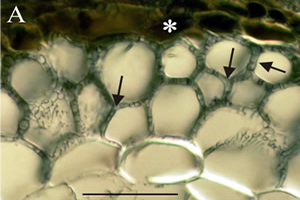
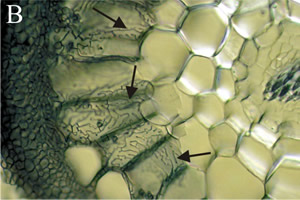
Ectomycorrhizas start to develop when hyphae infect the secondary or tertiary roots of woody species, which they seem to prefer, especially on trees. Hyphae grow back up the root from just behind the root cap and meristem, forming a weft, that may later become a bulky sheath. Hyphae from the sheath grow inwards between epidermal cells and cortical cells, by forcing their way mechanically and by excreting pectinases. These invasive hyphae form the Hartig net.
Hyphae never penetrate into cells or into the stele. The intercellular Hartig net may surround each cell completely, so they have no contact with any other cells. The extensive surface area of the Hartig net is the main interface for exchange of substances between plant and fungus. Fungal infection changes the growth pattern of the root. The fungal sheath reduces the rate of cell division at the root tip, slowing cell elongation and hence reducing the root growth lengthways. Cortical cells also elongate radially (shown in Fig. 3B) and so the infected root becomes short and thick compared to uninfected ones (Fig. 4). Hence, they are often called short roots. The fungal sheath also suppresses the development of root hairs, which essentially means that all nutrients entering the plant must be channelled through the fungal tissues of the sheath.
A fungus will extend its sheath with a growing root, ensuring the root is always covered, and colonisation by other fungi does not occur. However, fungi can be replaced as roots resume growth after dormancy. The fungal sheath may not resume growth straight away, and the root can be exposed to other fungal symbionts. This interactive replacement contrasts with the 'non-interactive' replacement mentioned before with reference to early and late stage colonisers. The advantages of the ECM association go beyond the mutual exchange of nutrients (click on the hyperlinked phrase for more information on nutrient exchange). Ectomycorrhizas can also confer pathogen resistance to their plant partner, and they are more effective at this, compared to AM roots. Click for more information on the effects of mycorrhizas. |
To skip to other mycorrhizal types, click below: |
|||
Close the window to return to your previous page |
|||