Nutrient exchange
The bi-directional movement of nutrients, carbon (C) from plant to fungus, and soil nutrients from fungus to plant, is the essential feature of a mycorrhiza, and is believed to be the basis for the mutualistic association. Aside from monotropoid mycorrhizas, the juvenile stages of all orchids, and the whole lives of a few orchid species (which are achlorophyllous) there is always a two-way transfer of nutrients.
As a rule, mycorrhizal infection enhances plant growth, by increasing nutrient uptake in up to three ways:
- increasing the surface area of absorption within the soil;
- by mobilising sparingly-available nutrient sources from unavailable compounds;
- by excreting chelating compounds or ectoenzymes.
 |
| Fig. 1. The effects of mycorrhizal inoculation on crop growth. Right-hand block inoculated with AM fungi, left-hand block not inoculated. Reprinted from Smith & Read (1997), Mycorrhizal Symbiosis. © Academic Press London. Reprinted by permission of the publisher. |
Path and site of exchange
Nutrients are obtained by hyphae, which extend far from the roots, and can therefore penetrate and exploit a larger volume of soil. From uptake by hyphae, nutrients are translocated within the hyphal network to the fungal sheath, intercellularly to the hartig net and with the exception of ectomycorrhizas and monotropoid mycorrhizas, intracellularly into plant cells.
The fungal sheath can act as a storage site for nutrients; allowing the fungus to continue to provide nutrients to the plant host when soil concentrations decrease.
In return for receiving extra nutrients, mycorrhizal plants loose between 10% and 20% of the photosynthates they produce, which go to the formation, maintenance and functioning of the mycorrhizal fungi and their associated structures.
With regard to the transfer of carbon from plant to fungus, labelled carbohydrates entering the fungi appear in the hyphae as trehalose, and in some strains as mannitol. When carbohydrates are transferred from fungus to plant, as in orchid and monotropoid mycorrhizas, the reverse occurs, with trehalose and mannitol being converted to sucrose.
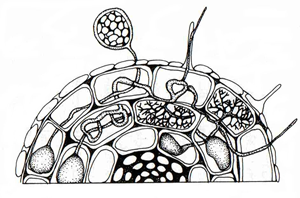
In AM mycorrhizas nutrient exchange occurs via the arbuscles. This is an intracellular interface, a situation also found in ericoid mycorrhizas. In contrast, the interface between plant and fungus in ectomycorrhizas takes place via the Hartig net, an intercellular structure of varying thickness.
 |
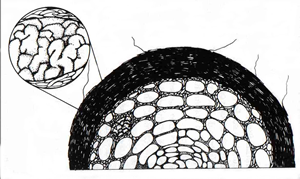 |
| Fig. 2. The AM interface (above left) and the ectomycorrhizal (ECM) interface (above right), where the exchange of nutrients occurs. In AM, exchange is via the intracellular arbuscles. In ECM, it is via the intercellular Hartig net. From Allen, M.F. (1992) Mycorrhizal Functioning. An Integrative Plant-Fungal Process. © Chapman & Hall, London. Reproduced by kind permission of Kluwer Academic Publishers. | |
This two-way exchange of nutrients in AM symbioses takes place between the plant root cortical cell and the fungal arbuscule that has penetrated it; this is the symbiotic interface and on the one side it is bordered by the plant plasma membrane and its extracellular matrix, and on the other side by the fungal plasma membrane and its extracellular matrix (Fig. 3).
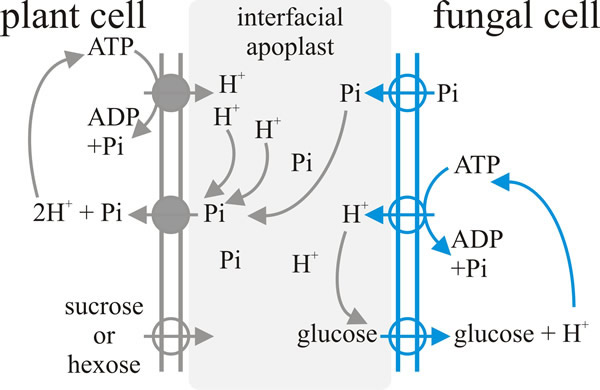 |
| Fig. 3. Schematic illustration of a model for the exchange-transfer of phosphate and carbon compounds across the arbuscular mycorrhizal interface. Plasma membrane proton-pump ATPases and secondary transporters that have been experimentally localised in the membranes of the arbuscular interface are indicated by the circles, with the arrows indicating direction of transport. Figure and caption from Moore et al., 2011 (URL). |
Nutrient transfer between fungus and plant is indirectly linked; that is, there is no ‘one-for-one’ linkage between, say, glucose and phosphate exchange.
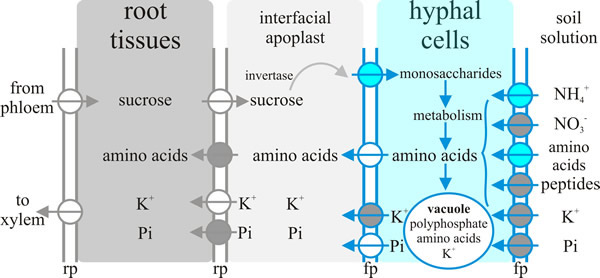
Arguably the most important communication between plant and fungus in the ectomycorrhiza is the exchange of nutrients. As with the exchange of nutrients between endomycorrhizas and their hosts (see discussion and Fig. 8, above) nutrient exchange between fungus and host depends on one partner releasing nutrient into the apoplastic interface and the uptake of that nutrient from the apoplastic interface by the other partner. A model showing the transporters either known or hypothesised to be involved in ectomycorrhizal tissues is summarised in Fig. 4. Note that the system operating at the plant-fungus interface, which is the Hartig net, involves:
- Delivery of sucrose into the apoplast by the plant and its hydrolysis by a plant-derived acid invertase; the resulting hexoses then being taken up by the fungal cells.
- Fungal Pi (inorganic phosphate) uptake systems harvesting Pi from the environment, probably assisted by several excreted fungal enzymes (like phosphatases) that could play crucial roles in improving P availability by digesting soil reserves of bound phosphate. The hyphae translocate P by active processes (highly mobile vacuoles?) to deliver to the apoplast for take up by their P-deficient host plant.
- Potentially nitrate, ammonium and peptide uptake into the fungus through specific fungal uptake systems feeding intermediary metabolism that supplies amino acids for release into the apoplast by the fungus through amino acid exporters, followed by subsequent amino acid uptake by the plant cell.
- High affinity active K+ uptake by peripheral hyphae from the soil solution and its release through fungal outward K+ channels into the apoplast for absorption by (currently hypothetical) host plant K+ importers.
 |
|---|
| Fig. 4. Nutrient exchange between fungus and host in the ectomycorrhiza depends on one partner releasing nutrient into the apoplastic interface and the uptake of that nutrient from the interfacial apoplast by the other partner. This diagram summarises current ideas about the transporters acting in ectomycorrhizal tissues that achieve this nutrient exchange. Key: fp, fungal plasma membrane, rp, root plasma membrane. The circles represent transporters, with the arrows indicating direction of transport. Blue circles represent transporters where at least one member of the transporter family has been characterised by functional complementation of a yeast deficient strain; grey circles are putative transporters for which candidate genes exist in the genome; white circles represent hypothetical transporters. Figure and caption from Moore et al., 2011 (URL). |
Rates of uptake
Direct uptake of nutrients by plant roots can often result in zones of nutrient deficiency around the root system. Subsequent absorption of nutrients is then limited entirely by the rate at which nutrients move through the soil. Hyphae can grow past these depletion zones and therefore maintain the rate of absorption by active translocation through the hypha. Maintaining and extending hyphae is a more economical way of sustaining contact with nutrients than by continually extending roots.
The rate at which nutrients move through the soil can be crucial to their availability. Phosphorus, in the form of phosphate (PO4-), has very low mobility in soil, and therefore tends to be the limiting nutrient in most ecosystems. In contrast, ammonium (NH4+) is about 10 times more mobile than phosphate, but is also required and absorbed in 10 times greater quantity.
Nitrogen
Nitrate (NO3-) is more mobile than ammonium, and is the main source of nitrogen in most agricultural soils (because of the application of artificial fertilisers). The exception to this rule is in acidic soils, such as those of forests and heathlands, where ammonium is in greater abundance. Most plants will preferentially take up ammonia over nitrate and research with ectomycorrhizal fungi has shown that many of these fungi may not be able to utilise nitrate. This is advantageous to plants, as even if ammonia is in low concentration, the ectomycorrhizal fungi will absorb it more rapidly than nitrate.
Ericoid mycorrhizas are particularly efficient at assimilating ammonium, since their partner plants are mostly heathland plants, which grow on the acidic soils which are high in that form of nitrogen. However, an important consideration is that nitrate (in particular) and ammonium salts are subject to leaching by rain water and this effect will keep their concentrations relatively low in the rhizosphere of most natural soils.
It is consequently extremely important that ectomycorrhizas and ericoid mycorrhizas can also utilise organic N sources, through the production of acid proteinases. This allows them access to N sources that are otherwise unavailable to the plant roots. In contrast, arbuscular mycorrhizal (AM) inoculation has little effect on N nutrition, and when nitrate is plentiful in the soil, AM plants have no advantage over non-AM plants. The rule of thumb seems to be that ectomycorrhizal and ericoid plants tend to dominate in N-limited soils, and AM dominate in P-limited soils.
Phosphorus
Phosphorus (P) is the limiting nutrient in most soils and it can be said that, in general, large growth increases due to mycorrhizal infection are primarily due to improvements in P absorption. When root growth is restricted in AM plants, up to 80% of the P used by the plant can come via the mycorrhizal hyphal network.
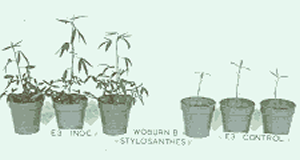 |
| Fig. 5. A classic mycorrhizal demonstration of enhanced growth by plants which have been inoculated by mycorrhizas (on the left), compared with the uninoculated control plants on the right. From Kendrick (1999) The Fifth Kingdom. © Mycologue Publications with permission. |
Acquisition of can be maintained by the fungus in three ways:
- forming polyphosphates in the hyphae, which maintains a low internal phosphate concentration (low Pi) and therefore a favourable concentration gradient allowing phosphate to passively diffuse into the hypha;
- small hyphal diameter maximises the surface area to hyphal volume ratio and consequently to a larger volume of soil being explored per unit surface area of hyphae;
- production of extracellular acid phosphatases, which release P from organic complexes in the soil. [AM fungi are actually unable to liberate P in this way and as with releaser of nitrogen from organic complexes, it is a strategy particularly well developed in ectomycorrhizas and ericoid mycorrhizas.
The beneficial effects of mycorrhizas with regard to P uptake are lost if the concentration of P in the soil increases. Both AM and ectomycorrhizal infection decreases on soils that have high phosphorus availability. In such soils, this may lead to the mycorrhizal association being abandoned, or can lead to growth depression; the fungus essentially shifts from mutualism to parasitism.
Monotropoid and Orchid mycorrhizas
The cases of monotropoid and the mycorrhizas of seedling orchids show different movement of nutrients between plant and fungus. The plants can be considered to be mycoheterotrophic, since they obtain their supplies of organic C from the mycorrhizal fungus, but give nothing back in return. Mineral nutrients are also supplied to the plants by their fungi.
However, the fungi appear not to be disadvantaged by this relationship as in monotropoid mycorrhizas, the fungus sequesters its carbon from other photosynthetic plants. Monotropoid mycorrhizas are often ectomycorrhizal with surrounding trees, and photosynthetic carbohydrates from that other plant host sustain both the fungus and the associated Monotropa sp. The monotropoid relationship has been referred to as epiparasitic, but since the fungus is not damaged in the relationship, this term has been dropped in favour of mycoheterotrophic. The fungus almost certainly receives nothing in return from Monotropa sp. although whether orchid mycorrhizal fungi receive photosynthetic carbohydrates in the adult stages of orchid growth remains to be demonstrated.
Find out more about the commercial applications of mycorrhizas and the effects of mycorrhizas by clicking on the hyperlinked phrases.
Further information about mycorrhizas can be found in the new textbook 21st Century Guidebook to Fungi by David Moore, Geoffrey D. Robson & Anthony P.J. Trinci. Published 2011 by Cambridge University Press: ISBN: 9780521186957. URL: ttp://www.cambridge.org/gb/knowledge/isbn/item6026594/?site_locale=en_GB. View Amazon page.
Updated December 15, 2016
