16.12 Clinical groupings for human fungal infections
There are five types of mycoses to describe, in two main categories:
- skin mycoses
- superficial mycoses
- cutaneous mycoses
- subcutaneous mycoses
- systemic mycoses
- systemic mycoses due to primary (usually dimorphic) pathogens
- systemic mycoses due to opportunistic pathogens.
An excellent online source of information about human fungal infections is the U.S. Department of Health & Human Services Centers for Disease Control and Prevention website at this URL: https://www.cdc.gov/fungal/diseases/index.html.
Superficial mycoses (or Tineas) mostly occur in the tropics and are restricted to the outer surface of the hair and skin. Examples are:
- Piedraia hortae, a filamentous member of the Ascomycota which causes black piedra, a disease of the hair shaft characterised by brown/black nodules on the scalp hair (actually the ascostromata of the fungus).
- Trichosporon cutaneum is a yeast belonging to the Basidiomycota that is common in soil, water samples, plants, mammals and birds, as well as being a member of the normal flora of mouth, skin and nails. It causes white piedra, a superficial infection of the skin, and scalp and pubic hair (although it is emerging as an opportunistic pathogen of the immunocompromised) (Taylor & Gurr, 2014).
Cutaneous mycoses. There are three genera of fungi that commonly cause disease in the non-living tissues of skin, hair, or nails/claws of people and animals, by growing in a zone just above where the protein keratin is deposited. These three genera are Microsporum, Trichophyton and Epidermophyton (all filamentous Ascomycota) and they are often labelled ‘dermatophytes’ (with the disease being called ‘dermatophytosis’) although, of course, they are not plants, so they can’t be any sort of ‘…phyte’ and a better term would be dermatomycosis. These fungi all have the ability to degrade keratin and grow as non-invasive saprotrophs on skin and its appendages, but their growth causes irritation and inflammation of underlying epithelial cells, this being an allergic reaction that may result in death of these cells (Table 2).
| Table 2. Examples of fungi that cause cutaneous mycoses, of which there are about 250,000 cases per year in the UK | |
| Fungus | Disease |
| Trichophyton rubrum | foot and body ringworm of man; infections of nail bed (onychomycosis); may spread to groin and hand |
| Trichophyton mentagrophytes var. interdigitale | Athletes foot of man, mainly in the web of skin between toes and lower surfaces of toes; favoured by humid conditions and common in shoe wearing people; can also affect hand and can cause onychomycosis; common in the fur of rabbits and rodents |
| Trichophyton verrucosum | infection in the fur of cattle and horses but can be transmitted to man; can lead to permanent baldness and pitting of scalp skin |
| Microsporum audouinii | ringworm in children; tends to be restricted to man as host; common in underdeveloped countries; the fungus forms rings in the skin because they the mycelium grows like colonies in Petri dish cultures (and the redness is an inflammatory response to proteins secreted by the fungus) |
| Microsporum canis | common in the fur of dogs and cats where it can cause ringworm; often acquired by children in contact with domestic animal pets |
| Microsporum gypseum | can be isolated from soil and cause cutaneous mycoses; other ‘dermatophytes’ are poor competitive saprotrophs and do not survive in soil |
| Epidermophyton floccosum | Man is the primary host of this, the only pathogenic species of Epidermophyton, infecting skin, causing tinea corporis, tinea cruris, tinea pedis, and nails, causing onychomycosis. |
| Transmission of dermatophytes: physical contact between hosts, spores become lodged in small wounds, mycelium may persist in sloughed off skin scales or detached hairs, shared facilities such as swimming pools, schools and colleges, ‘petting zoos’, may act as a focus of an epidemic. | |
Infections of human finger and toenails are so important that they have their own descriptive term: onychomycosis, which is used to refer to nail infections caused by any fungus. Several ‘dermatophytes’ cause onychomycosis (see Table 2), and non-‘dermatophyte’ causes include Scopulariopsis brevicaulis (filamentous, Ascomycota) and Candida albicans (yeast, Ascomycota). It is not uncommon to have more than one fungus species jointly causing the infection.
Nearly 1.8 million people in the UK suffer from ‘nail fungus’ and nail infections can be extremely distressing to the affected individuals. Prevalence varies from 2 to 3% (USA) to 13% (Finland), the disease is twice as frequent among men as women, and it increases with age; rates increasing to about 25% among elderly patients. Prevalence is also high in patients with diabetes and immunosuppressed individuals.
The fungi that cause cutaneous mycoses occur widely in nature and can be:
- geophilic, inhabiting the soil as decomposer saprotrophs and causing infections following contact with soil (for example, Microsporum gypseum);
- zoophilic, primarily parasitic on animals, but infection is transmitted to man following contact with the animal host (for example, Microsporum canis, Trichophyton mentagrophytes and Trichophyton verrucosum);
- anthropophilic, primarily parasitic on man, rarely infecting animals (for example, Microsporum audouinii, Trichophyton rubrum, Trichophyton tonsurans, and Epidermophyton floccosum).
Subcutaneous mycoses are generally caused by fungi that are normally saprotrophic inhabitants of soil, particularly in tropical and subtropical areas of Africa, India, and South America, which become infective by being introduced through wounds in the skin. Most infections involve people who normally walk barefoot.
- Madurella mycetomatis and M. grisea (filamentous, Ascomycota) cause human mycetoma (common name: madura foot), which is a localised infection causing locally invasive tumour-like abscesses, accompanied by chronic inflammation, resulting in swelling, distortion and ulceration of the infected body part. The fungus is introduced through mild wounds in the skin and may grow for several years in the cutaneous and subcutaneous tissues, extending to connective tissues and bones. Mycetomas are usually resistant to chemotherapy, leaving surgery, even amputation, as the only resolution.
- Sporothrix schenckii (thermally dimorphic, Ascomycota) causes sporotrichosis. Sporothrix is the anamorph and Ophiostoma stenoceras the teleomorph. The fungus occurs in soil worldwide although the disease is localised, with Peru having the highest prevalence of Sporothrix schenckii infections. Also called ‘rose handler’s disease’, sporotrichosis starts by entry of the fungus through minor skin injury and can then spread through the lymphatic system. The fungus is dimorphic: forming septate vegetative hyphae, conidiophores and conidia at 25°C, while at 37°C oval to cigar-shaped budding yeast cells are produced. As the yeast form is distributed by the lymphatic system, disseminated sporotrichosis can result in infections of the lungs and bones and joints, endophthalmitis (inflammation of the internal layers of the eye), meningitis and invasive sinusitis.
Systemic mycoses are infections that affect the whole body. We divide these into mycoses due to primary (usually dimorphic) virulent pathogens, and those due to opportunistic pathogens. There is potential for overlap in these two categories. They are all deep seated mycoses that can affect the whole body, but some opportunistic pathogens are dimorphic, some are inhaled. The key difference is in the point that we have already stressed: primary pathogens are virulent and generate a disease infection as soon as they encounter the host; opportunistic pathogens are usually saprotrophic or commensal organisms that become pathogenic when the opportunity offered by a weakened immune system is presented.
Systemic mycoses due to primary pathogens are usually acquired by inhalation of virulent spores originating from soil or related substrata, so the disease starts off primarily in the lungs and can spread to other organ systems. The four prime examples are blastomycosis, coccidioidomycosis, cryptococcosis and histoplasmosis.
Blastomycosis is caused by the dimorphic fungus Blastomyces dermatitidis (Ascomycota), which is endemic as a saprotroph of decaying woody material in the soil of the Mississippi and Ohio River basins in the USA. Dimorphism is temperature dependent; conversion of the filamentous hyphal growth form to a budding yeast occurs at 37°C. Outbreaks of the disease are associated with occupational or recreational activities around streams and rivers, and wet soils containing rotting organic woody debris. Infection results from inhalation of conidia, which germinate into the yeast form in the lungs. After 30 to 45 days an acute pulmonary disease indistinguishable from a bacterial pneumonia occurs, although the disseminated disease can affect the skin, genitourinary tract and other organs as well as the lungs. On average there are about 30 to 60 deaths due to blastomycosis in the USA each year.
Coccidioidomycosis, or ‘Valley fever’, is caused by thermally dimorphic fungal species of the genus Coccidioides, which are found (in rodent burrows) in alkaline soil of warm, dry, low altitude areas with high summer temperatures. There are only two species in the genus: Coccidioides immitis and C. posadasii which co-exist in the desert southwest of the USA and Mexico while C. immitis is geographically limited to the San Joaquin valley region of California, and C. posadasii is also found in South America. The division into two species was published in 2002 and before this the two were known as the California and non-California variants of C. immitis. Inhalation of the dry arthroconidia of Coccidioides, which are carried by dust storms, is the infection mechanism. Coccidioidomycosis is initially a lung infection; the young and healthy may suffer a slight cough, but it clears up. If the victim is not in the best of health, the infection can spread to skin, bones, joints, lymph nodes, adrenal glands, and central nervous system. The disease may prove fatal; it causes about 50 to 100 deaths per year across North, Central and South America.
Cryptococcosis is caused by Cryptococcus neoformans (a basidiomycete encapsulated yeast); the environmental sources of which are soil and other habitats (trees, buildings, etc.) contaminated with pigeon droppings. Though it has a worldwide distribution it is predominantly a disease of Northern Europe. The infection starts with inhalation of the organism and can remain localised in the lungs but often disseminates throughout the body and the most severe form is probably the non-contagious cryptococcal meningitis when the fungus reaches the brain. Cryptococcosis can be considered an opportunistic infection as it mainly affects immunosuppressed patients, being found in about 15% of AIDS patients (the annual incidence before the AIDS epidemic has been estimated at 2-9 cases per million inhabitants; following the emergence of AIDS, the annual incidence of cryptococcosis in the early-to-mid 1990s varied between 17 to 66 cases per thousand persons living with AIDS (Taylor & Gurr, 2014).
Histoplasmosis exists in two forms; both of which are caused by varieties of Histoplasma capsulatum. The most common is the pulmonary disease known as North American Histoplasmosis caused by Histoplasma capsulatum var. capsulatum. The other form has been called African Histoplasmosis, caused by Histoplasma capsulatum var. duboisii, and is usually a disease of the skin and bones. Histoplasma (Ascomycota) is a thermally dimorphic anamorph (grows filamentously at 25°C, as a budding yeast at 37°C), which has a teleomorph with the name Ajellomyces capsulatus. Soil contaminated with bird or bat droppings is the common natural habitat of Histoplasma, which has a worldwide distribution although the disease occurs in tropical areas. Histoplasmosis is endemic in the Tennessee-Ohio-Mississippi river basins of the USA where it causes about 50 deaths per year.
Systemic mycoses due to opportunistic pathogens are infections caused by fungi with low inherent virulence, which an almost limitless number of fungi common in any or all environments. Health statistics indicate the sinister fact that mortality from fungal disease has been steadily increasing since the 1980s against a background of steady decline in mortality caused by all other infectious agents. There are a number of identifiable reasons for this. There has been an increase in clinical awareness leading to improved diagnosis of fungal disease (that is, not necessarily more disease, but more of what does occur is being diagnosed as being of fungal origin). To some extent this reflects the introduction of techniques, especially molecular methods, which can rapidly identify fungi. Secondly, increased availability of international travel has taken more people into the tropics, and tropical regions do seem to harbour more fungal pathogens. Thirdly, and above all, there has been an increase in the number of immunocompromised patients. Drug therapies used to manage the immune system in transplant and cancer patients have the unfortunate side effect of weakening the body’s defences against fungal pathogens, and AIDS sufferers have similarly weakened immune defences against fungi. Indeed, it is likely that most AIDS-related deaths are ultimately due to fungal diseases. The fungi most frequently isolated from immunocompromised patients are the endogenous commensal diploid yeast Candida and saprotrophic (that is, environmental) filamentous Aspergillus and zygomycete species. As we will point out, the spectrum of fungal species responsible for these diseases is continually, and quite rapidly, changing.
Candidiasis. Candida species have the ability to colonise any surface of the human body and grow without causing disease; when the normal host defence mechanisms of an individual are impaired the fungus can become pathogenic. Candida albicans is a component of the normal microbiota of the human body. It normally exists in ecological balance with other microbes and you don’t know it’s there; however, steroid or immunosuppressive drug treatments, and particularly antibacterial antibiotics, reduce the other microbes and consequently increase the opportunities for Candida (Table 3).
| Table 3. Isolation of Candida albicans from untreated and antibiotic treated individuals | |||
| Patient category | %age frequency of isolation from |
||
mouth |
alimentary tract |
vagina |
|
| Disease free individuals | 43 |
15 |
8 |
| Individuals given anti-bacterial antibiotics | 76 |
22 |
15 |
In fact, a decrease in bacterial populations increases the chances of C. albicans adhering to the gut lining; indigenous bacteria suppress adherence of C. albicans, and adhesion to epithelial surfaces is an important aspect of C. albicans pathogenicity. Candida most commonly forms superficial lesions on the mucous membranes of the mouth and vagina. The disease of the mouth, known as ‘thrush’, occurs on the tongue, gums and buccal mucosa, commonly in infants, and in adults with hormonal or immunological problems. The disease of the vagina, vaginitis, causes itching and smarting of lower vagina and is the second most common infection in the UK. Up to 75% of women suffer at least one episode, with half of them suffering a further episode. It is often associated with pregnancy, when the pH of the vagina is low, and the incidence is increased by use of steroid based contraceptives.
Mucosal candidiasis occurs in almost all immunocompromised AIDS sufferers, and is also common in organ transplantation patients and those undergoing anti-cancer therapies because of the immunosuppressive effect of the therapies. Defects in cell-mediated immunity render AIDS patients particularly susceptible to fungal infections; 60-80% suffer at least one fungal infection and at least 10-20% will die as a result of fungal infection.
Candida is usually described as a dimorphic fungus, although it would be better described as pleomorphic with a range of morphologies, from ovoid yeast cells at one extreme to filamentous hyphae at the other (Odds, 2000). When C. albicans is grown in medium containing serum it forms pseudo-hyphal germ tubes, not buds. This is used as a diagnostic feature. The hyphal (or pseudo-hyphal) phase has several advantages over the yeast form because it is:
- more pathogenic than the yeast phase,
- more efficient at penetrating epithelial layers, and
- more resistant to defence systems.
C. albicans has been regarded as the most common cause of invasive yeast infection for a long time. It responds well to treatment with fluconazole and as a result the overall incidence of infections due to C. albicans has decreased. However, since the early 1990s, infections caused by Candida glabrata have increased, and this species is less susceptible to fluconazole and has also been associated with oropharyngeal candidiasis in patients receiving radiotherapy for head and neck cancer (Nucci & Marr, 2005).
Candida albicans and Cryptococcus neoformans are both capable of rapid phenotypic switching, where genetically identical cells can exist in two distinctive cell forms: white and opaque in the case of C. albicans. The white cells exhibit the classic yeast cell shape, while the opaque cells are elongated and have a distinctive sculptured surface. Each cell type is stably inherited for many generations and switching between the two types of cells occurs rarely and randomly. This phenotypic switch is known as an epigenetic phenomenon in the sense that changes in gene expression are heritably maintained without any modification to the primary genomic DNA sequence. A highly interconnected network of sequence-specific DNA-binding proteins control this switch (Whiteway & Oberholzer, 2004; Hernday et al., 2016). The two types of cells interact differently with their mammalian host, with opaque cells being more suited to skin infections, and white cells better suited to blood stream infections. Odds (2000) suggests that ‘switching’ could represent a mechanism of accelerated micro-evolution to assure survival of the type that can most rapidly adapt to a new microniche.
Aspergillosis is a disease of the lungs caused by inhaling conidia of Aspergillus spp. It occurs in mammals and birds and was the first bird mycosis to be identified (at the beginning of the 19th century) and can be epidemic in chickens reared under crowded conditions. Aspergillus has a worldwide distribution and its saprotrophic lifestyle enables it to be a constant component of our way of life. Aspergillosis has been reported in almost all domestic mammals and birds as well as in numerous wild species. The species most commonly associated with aspergillosis are Aspergillus fumigatus. A. flavus, A. niger, A. nidulans, A. terreus, and A. glaucus, which are common and widespread, growing on all manner of organic debris.
Aspergillus species can produce enormous numbers of spores and they are common moulds in nature; their spores can heavily contaminate hay, straw and grain workers who regularly handle these products (farm and brewery workers) were the groups at greatest risk of ‘farmer’s lung disease’ up to about the middle of the 20th century when the disease was a rare occupational hazard. Aspergillosis in humans is principally (>90%) caused by Aspergillus fumigatus and the greatest risk group is to patients being treated for cancer or with immunosuppressive therapies after organ transplantation who suffer neutropenia (reduced neutrophil levels). This type of immune suppression is not usually seen in people with HIV, so aspergillosis is rare among HIV-positive patients. Aspergillus spp. cause a spectrum of respiratory diseases as the inhaled spores become lodged in the lungs and bronchi (Fig. 11).
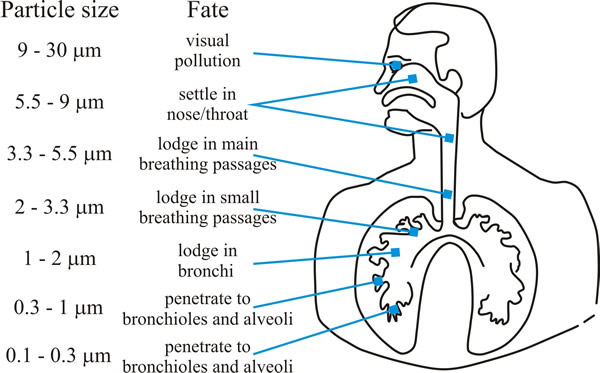 |
| Fig. 11. Graphic showing how deeply particles can be inhaled into the human respiratory tract. Aspergillus conidia are in the size range (2-4 µm) that enables them to penetrate deeply into the human lung. |
Allergic aspergillosis is an immediate reaction to the spores that leads to asthmatic reactions. Further damage causes a bronchitis called bronchopulmonary aspergillosis. Ultimately ‘colonising aspergillosis’ occurs where the fungal hyphae grow into ‘fungal balls’ (aspergillomas) that are formed within the natural lung cavities. Finally, invasive and disseminated aspergillosis spreads from the pulmonary focus to a variety of tissues and organs. The disease was rare before the use of immunosuppressive drugs became common. Corticosteroids are anti-inflammatory medicines that are prescribed for a wide range of conditions, but they are associated with an increase in invasive aspergillosis because corticosteroids impair immune function in mammals and, specifically, the ability of human macrophages to destroy conidia. Hydrocortisone is a corticosteroid that is widely used to treat numerous human ailments. Unfortunately, clinically effective doses have been shown to cause a 30 to 40% increase in growth rate of A. fumigatus and A. flavus (Ng et al., 1994) and patients receiving corticosteroid therapy had significantly increased levels of Aspergillus and total fungus in their lungs (Fraczek et al., 2018).
Although virtually everyone is exposed to Aspergillus in their daily environment, healthy respiratory tracts and healthy immune systems deal effectively with inhaled spores and it rarely causes disease. Historically, it was a problem only in those few occupations that exposed workers to such massive spore concentrations that their healthy respiratory defences were overwhelmed, and respiratory disease resulted.
Today, fungal diseases are a serious health problem worldwide and Aspergillus fumigatus and Aspergillus flavus are the most common causes of invasive mould infections and carry a high mortality. Therapeutic suppression of the immune system is associated with aspergillosis causing serious lung problems and the infection can also spread to the kidneys, liver, skin, bones, and brain. Because these more severe forms of aspergillosis are almost always fatal (Table 4), it is important to diagnose and treat this infection quickly (see the Aspergillus Website at https://www.aspergillus.org.uk/; the Support for People with Aspergillosis website at https://www.nacpatients.org.uk/; and the National Aspergillosis Centre http://www.nationalaspergillosiscentre.org.uk/).
| Table 4. Outcomes for patients treated for invasive aspergillosis | |
| Type of aspergillosis | % Overall mortality |
| Disseminated (disease at multiple sites) | 95-100 |
| Cerebral aspergillosis | 95 |
The epidemiology of mould infections changed substantially in the 10 years from 1995. The incidence of invasive aspergillosis increased significantly, and infections caused by fungi showing resistance to conventional antifungal agents, such as Fusarium spp. (Ascomycota) and zygomycetous species have also increased. Nucci & Marr (2005) describe this as evolution of the epidemiology of invasive fungal infection, and show that yeasts other than Candida albicans and filamentous fungi other than Aspergillus fumigatus are emerging as significant causes of invasive mycoses in severely immunocompromised patients.
For example, in one transplantation clinic A. terreus accounted for 2.1% of cases of invasive aspergillosis in 1996 and 10.2% of cases in 2001. Aspergillus terreus is a concern because of it shows resistance and poor clinical response to what has been the prime antifungal agent, amphotericin B. Infections caused by newly recognised species of Aspergillus (‘Aspergillus lentulus’) have also appeared, isolates of which have low susceptibility to multiple antifungals in vitro. The reasons for these changes are a complex mixture of altered prophylactic use of antifungal agents and altered surgical procedures that together change the environment that the infecting fungus encounters in organ transplant recipients, premature newborns, and other critically ill patients (Nucci & Marr, 2005). A novel class of antifungals, the orotomides, is currently in clinical development for the treatment of invasive aspergillosis to overcome this growing problem of resistance to current therapies (Oliver et al., 2016; du Pré, et al., 2018).
Pneumocystosis is the overall name given to infections caused by Pneumocystis, which have four clinical expressions: asymptomatic infections, infantile pneumonia (and may be associated with sudden infant death syndrome (SIDS)), pneumonia in immunocompromised patients, and extrapulmonary infections. Pneumonia due to Pneumocystis is frequently written with the acronym PCP (PneumoCystis Pneumonia) and is one of the most important pneumonias in immunocompromised individuals. Pneumocystis was classified as a protozoan until the late 1980s but is now clearly accepted as a yeast-like fungus and molecular phylogenies place it in the Ascomycota, Taphrinomycotina (see Chapter 3; CLICK HERE to view the page). The original specific name given to the organism was Pneumocystis carinii but isolates causing infections in humans were misidentified and are now referred to P. jirovecii in honour of the Czech parasitologist Otto Jirovec. Pneumocystis carinii is still the correct name for this organism when found in hosts other than man.
Pneumocystis DNA can be detected in air and water, although the organism may not be visible microscopically, suggesting that Pneumocystis may not survive in the environment longer than it takes to infect a susceptible host. The organism cannot be cultured in vitro, so the only information about the life cycle comes from studies in animals. Pneumocystis trophic (vegetative) forms are produced during asexual growth; this is called a trophozoite, the terminology surviving from the time the organism was thought to be a protozoan. Trophozoites are variable in morphology and occur clustered together in the host tissue. They are probably haploid and capable of replicating asexually by mitosis, and they produce a diploid zygote by conjugation. Meiosis occurs in the zygote, forming a precyst initially, then an early cyst and, finally, a mature cyst (again, terminology surviving from protozoan days); during this maturation process eight intracystic spores or ‘daughter cells’ are produced, which must be ascospores resulting from meiosis followed by a mitosis. These spores are released when the mature cyst ruptures and germinate into trophic forms. How this cycle relates to release of an infective agent to the environment is unknown. Sources of human infections are patients suffering pneumocystosis or immunocompetent individuals transiently parasitised by Pneumocystis (carriers); so Pneumocystis is unusual among pathogenic fungal species in that the sufferers somehow transmit the disease.
Pneumocystis is one of the major causes of opportunistic mycoses in immunocompromised patients, including those with congenital immunodeficiencies, AIDS, and patients receiving corticosteroid or intensive immunosuppressive therapy for treatment of cancer or prevention of transplant rejection. PCP has been in decline following the introduction of highly active antiretroviral therapy for HIV infections in 1996 (Sax, 2001; Hammer, 2005), but it is still one of the most significant AIDS-related diseases; in particular, extrapulmonary infections, resulting from dissemination of the infection from lungs to lymph nodes, spleen, bone marrow, liver, kidneys, heart, brain, pancreas, skin, and other organs, occur in patients with AIDS.
Zygomycosis is another emerging disease that is becoming an increasingly significant infection in transplant recipients. One survey of fungal infections in transplant recipients reported the proportion of cases of zygomycosis increased from 4% to 25% between 2001 and 2003. Analysis of risk factors associated with zygomycosis, compared with those associated with invasive aspergillosis, revealed an association between the use of voriconazole therapy and zygomycosis. Voriconazole is a triazole antifungal used to treat or prevent invasive candidiasis and invasive aspergillosis. Zygomycosis is considered the ‘third threat’ of fungal infection in patients who have survived infections caused by C. albicans and A. fumigatus (Nucci & Marr, 2005).
The most common species causing zygomycosis are Absidia corymbifera, Rhizomucor pusillus, and Rhizopus arrhizus, which are all commonly found in soil but can cause this acute and rapidly developing disease in debilitated patients. The disease is associated with acidotic diabetics, malnourished children, and severely burned patients, and also occurs with leukemia, lymphoma, AIDS, and immunosuppressive therapy. The infection typically involves the rhino-facial-cranial area, lungs, gastrointestinal tract, or skin; with the fungi growing in arterial blood vessels, causing embolisms and necrosis of surrounding tissue. Rhinocerebral zygomycosis in acidotic diabetic patients usually results in death within a few days.
Fusariosis in immunocompromised patients has also shown a rising trend. The most virulent Fusarium spp. is Fusarium solani (filamentous, Ascomycota). Fusarium is a well known plant pathogen and is widely distributed on plants, including crop plants, and in the soil. The rate of infection in haematopoietic stem cell transplantation (HSCT) recipients has increased over time. HSCT is now the second most frequent major organ transplant, being used to treat a variety of malignancies and bone marrow failure disorders; an estimated 45,000 such transplants are performed each year in the USA alone, 2000 in patients under 20 years of age. This population of patients could be at risk of fusariosis because of severe T cell–mediated immunodeficiency resulting from severe immunosuppression over long periods of time; some cases are diagnosed very late after transplantation (10 years and over) (Nucci & Marr, 2005).
The real worry now is the increasing resistance to the limited collection of antifungal drugs; especially for Candida and Aspergillus infections, for which the therapeutic options have become reduced. The emergence of multiply-drug resistant Candida species is a global health threat, and azole-resistant Aspergillus has up to 30% prevalence in some European hospitals, which report higher than 90% mortality rates (Fairlamb et al., 2016; Meis et al., 2016; Robbins et al., 2017). Remember that July 2017 editorial in the journal Nature Microbiology? Stop neglecting fungi (Anonymous Editorial, 2017), and the Royal Botanic Gardens’ report State of the World’s Fungi 2018 (Willis, 2018).
Updated March, 2020
