16.5 Entomogenous fungi
Entomogenous means ‘growing on or in the bodies of insects’ and strictly-speaking applies to all those organisms already discussed in this Chapter. However, the word is most often used to describe filamentous fungi that invade their insect hosts by penetrating directly through the cuticle, whereas most organisms in the groups we have already described are either ectoparasites or insect pathogens that generally infect the host when infective spores are ingested into the intestine.
In entomogenous (or ‘entomopathogenic’) fungi (Table 1) the general infection cycle is that a fungal spore adheres to the cuticle and germinates when conditions are suitable.
| Table 1. Examples of entomogenous fungi | ||
| Fungus | Taxonomic position | Host |
| Coelomomyces psorophorae | Blastocladiomycota | Alternates between the larvae of the mosquito (Culiseta inornata) and the copepod, Cyclops vernalis |
| Erynia | Zygomycota: order Entomophthorales | Erynia neoaphidis is a pathogen of aphids; Erynia radicans has potential to biocontrol the eastern spruce budworm |
| Entomophthora muscae | Zygomycota: order Entomophthorales | Adult Diptera, including houseflies |
| Verticillium lecanii now called Lecanicillium lecanii | Ascomycota: Hypocreales | Aphid, whitefly, used in production of Vertalec (used against glasshouse aphid) and Mycotal (used against glasshouse whitefly). |
| Beauveria bassiana (the anamorph, asexually reproducing form, of Cordyceps bassiana) | Ascomycota: Hypocreales: Clavicipitaceae | Termites, whitefly, different beetles (including potato pest Colorado Beetle) and might biocontrol the malaria-transmitting mosquito |
| Cordyceps militaris | Ascomycota: Hypocreales: Clavicipitaceae | Parasitises and kills moth pupae, including silkworm (Bombyx mori). Used as a traditional remedy in Asian folk medicine. |
| Nomuraea rileyi | Ascomycota: Hypocreales: Clavicipitaceae | Inhibits larval moult and metamorphosis in the silkworm (Bombyx mori) |
| Metarhizium anisopliae | Ascomycota: Hypocreales: Clavicipitaceae | Infects 200+ insect species, including grasshoppers, termites, thrips and might biocontrol the malaria-transmitting mosquito |
The germ tube of the spore then penetrates the host cuticle with a combination of enzymes and physical force and enters the body cavity (haemocoel or haemolymph) of the insect host (diagrammed in Fig. 7, with an indication of the insect defences) (Butt et al., 2016).
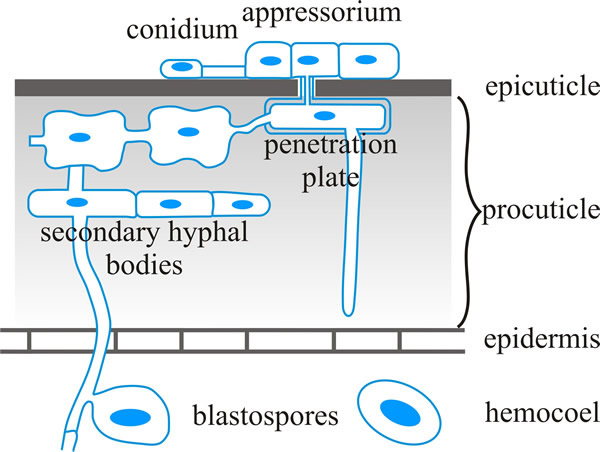 |
| Fig. 7. Schematic representation of infection structures of Metarhizium anisopliae (Ascomycota). This is a fungus, which is found in soils throughout the world, that causes disease in over 200 insect species. The illustration shows penetration of the insect cuticle in a diagrammatic cross-section. Cuticular resistance barriers may be (a) preformed (including those at the surface, like hydrophobicity, electrostatic charges, other microbes on the surface, low relative humidity and low nutrient availability, toxic lipids and phenols, and/or tanned proteins in the epicuticle; and tanned proteins with crystalline chitin to stiffen and desiccate the procuticle, and protease inhibitors in the procuticle), and/or (b) induced, such as melanisation which cross links cuticle components to provide a measure of resistance to fungal hydrolytic enzymes. Based on a figure in Hajek & St. Leger, 1994. |
Within the body cavity the fungus grows vegetatively, often in the form of yeast-like hyphal bodies that reproduce by budding, eventually filling the haemocoel and sending hyphae into the solid tissues of the host. As a result, the fungus depletes the tissues of nutrients and the host suffers starvation and may also be poisoned by toxins produced by the fungus. Eventually, the insect dies and the host cuticle ruptures (see the flow-chart summary in Fig. 8).
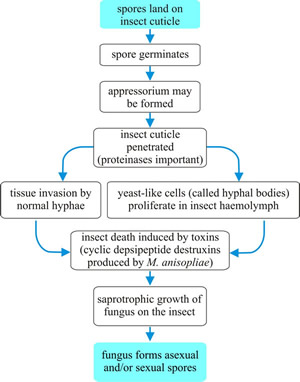 |
Fig. 8. Flow chart summarising the generalised infection process of entomogenous fungi. |
The fungus continues to grow saprotrophically on the cadaver and the dead insect becomes filled and covered with mycelium; fruiting/sporing structures emerge from the cadaver and generate infectious spores (shown in flow-chart summary in Fig. 8; photographs, Fig. 9) (Castrillo, Roberts & Vandenberg, 2005).
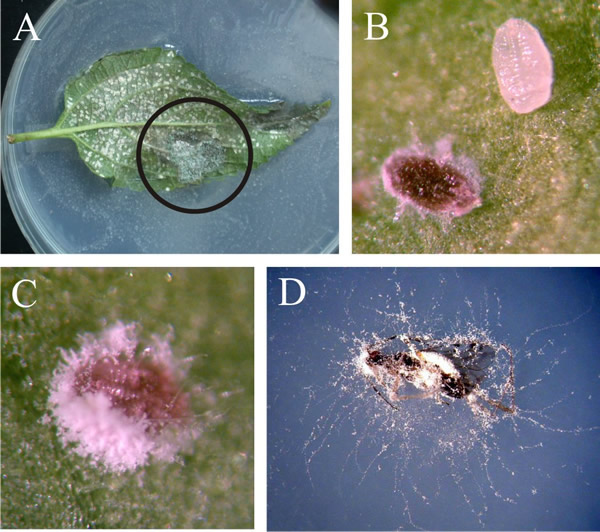 |
| Fig. 9. The final stages of Beauveria bassiana (Ascomycota) infections. A, B and C show the effect of B. bassiana infection on whiteflies, Trialeurodes vaporariorum. A shows the lower surface of a leaf heavily infested with whitefly on which many pupae have been infected by Beauveria (circled region in particular). B is a magnified view of the leaf surface showing a comparison between an infected whitefly pupa and a healthy one. The infected pupa is red due to the accumulation of large amounts of the red antibiotic oosporein, which is the major secondary metabolite excreted by Beauveria and several other fungi. Oosporein is an antifungal agent; is also antagonistic to Phytophthora infestans, and is a mycotoxin that causes skeletal problems in poultry fed contaminated grain. C is another whitefly cadaver showing conidiospore production. D is a cadaver of Dicyphus hesperus, which is used as a predator in biocontrol of whiteflies and spider mites in greenhouses but is also sensitive to Beauveria infection. Photographs by Roselyne Labbé. Modified from Labbé, R. (2005). Intraguild interactions of the greenhouse whitefly natural enemies, predator Dicyphus hesperus, pathogen Beauveria bassiana and parasitoid Encarsia formosa. M.Sc. thesis, Faculté des Sciences de L’Agriculture et de L’Alimentation, Université Laval, Canada at http://archimede.bibl.ulaval.ca/archimede/files/4ee0acbe-a906-4104-9589-3ee49c48d09b/22512.html, and see Labbé et al., 2009. Photographs kindly supplied by Dr Roselyne M. Labbé, Agriculture and Agri-Food Canada. |
This final stage is not seen in a few fungal pathogens that rely on dispersal by insect flight or other movement of the infected hosts, for example Massospora (Entomophthorales, ‘Zygomycota’), which causes a fungal disease that affects the Cicada, and Strongwellsea spp. (Entomophthorales, ‘Zygomycota’) which are common fungal pathogens of different types of adult flies. However, an insect cadaver covered by fungal mycelium from which fruit bodies are emerging and/or around which lays a carpet of released spores is the stage most commonly observed in the field. Because they are easily visible to the naked eye, observation of these final stages allowed early observations of fungal disease in commercially-valuable insects like the honey bee and silkworm, and helped give birth to invertebrate pathology as a field of study.
Entomogenous fungi have been described from all the major fungal phyla: chytrids, zygomycetes, Ascomycota, and Basidiomycota and over 700 species of fungi are known to infest insects. The zygomycetes and Ascomycota contain some extremely common insect pathogens that are also useful in biocontrol programmes (see next section, below). Although some fungi infect a range of insects (for example, Verticillium lecanii infects aphids, thrips and whitefly), other species can be extremely specific (Erynia neoaphidis only infects aphids). The infection process frequently involves specific fungus-insect host recognition interactions that can even be strain-specific for both fungus and host (Wraight et al., 2007) .
For example, two closely related species of Coelomomyces (Blastocladiomycota), C. dodgei and C. punctatus, have sporangia with similar sizes, shapes, and general morphology, and their host (mosquito) species ranges and geographic distributions overlap; but they do not interbreed and experimentally-forced hybrid zygotes were only partially viable, giving biological support to their classification into two distinct morphospecies (Castrillo et al., 2005).
The initial attachment step can be non-specific, involving passive hydrophobic interactions between the insect cuticle and hydrophobin proteins on the surface of the fungal spores (see the section entitled On the far side in Chapter 6; CLICK HERE to view the page). Spores of the common entomogenous fungi Beauveria bassiana, Metarhizium anisopliae and Nomuraea rileyi are like this and will adhere to both host and non-host insects.
A mucilaginous coat also permits passive attachment to surfaces in members of the Entomophthorales. In some aquatic entomogenous fungi initial contact between fungal zoospores and mosquito larvae is due to the zoospores having a negative geotaxis that favours collisions with mosquito larvae near the water surface. In contrast, selective attachment depends on the spore (or germ tube) interpreting chemical and physical cues on the host cuticle; this applies to the attachment to mosquito larvae of some species of the chytrid Coelomomyces.
In terrestrial filamentous fungi, spore germination results in the emergence of a penetrating germ tube, or a germ tube and appressorium. In either case a narrow penetration peg then breaches the insect cuticle using mechanical (turgor pressure) and/or enzymic means, particularly proteases in the latter case because insect cuticle comprises up to 70% protein, but including chitinases and lipases (Charnley, 2003; Pereira et al., 2007). Many spores also secrete mucilage during the formation of infective structures that enhances adhesion to the host cuticle. Appressorium formation and production of cuticle-degrading proteases are triggered by low nutrient levels in M. anisopliae, demonstrating that the fungus senses environmental conditions and host cues at the beginning of the infection process. The enzymes are produced in sequence; protease followed by chitinase is needed to dissolve the insect cuticle. An important point is that cuticle-degrading enzymes not only aid penetration but also provide the fungal germ tube with nutrients (Castrillo et al., 2005).
Entomogenous fungi are dimorphic (see the section entitled Yeast-mycelial dimorphism in Chapter 5; CLICK HERE to view the page) and after penetration the fungus changes from filamentous hyphal growth to growing as yeast-like or protoplast hyphal bodies that circulate in the haemolymph and multiply by budding. Later, the fungus changes back to a filamentous mode of growth to invade internal tissues and organs. In Entomophthora hyphal growth morphology was induced by low-nutrient conditions, particularly low nitrogen availability (Castrillo et al., 2005). The value of their filamentous morphology to entomogenous fungi is worthy of specific comment. Fungal pathogens of plants and insects have evolved similar methods of breeching the surfaces of their hosts, that is, they both form appressoria from which narrow hyphae penetrate the host’s surfaces by a combination of mechanical pressure and enzymatic softening. Filamentous fungi are far more successful than unicellular bacteria as pathogens of plants; and the same is true for insects. The reverse applies to their comparative success as pathogens of higher animals.
During growth in the host the fungi also produce a wide variety of toxic metabolites, which vary from low molecular weight products of secondary metabolism to complex cyclic peptides and enzymes, some of which are insecticidal. Few compounds have been found in diseased insects in sufficient quantity to account for disease symptoms. An exception is a family of cyclic peptides called the destruxins. These are cyclic depsipeptide toxins from Metarhizium spp. (a depsipeptide is a peptide in which one or more of the amide (-CONHR-) bonds are replaced by ester (-COOR) bonds). Twenty-eight different destruxins have been described with different levels of activity against different insects. The amount of destruxin produced correlates with virulence and host specificity. Destruxins modulate the host immune system and inhibit phagocytes (Charnley, 2003; Liu & Tzeng, 2012).
Many other toxins are produced by entomopathogenic fungi; for example, the entomogenous fungus Entomophthora muscae, which infects and kills domestic flies (Musca domestica), produces pheromones which attract other flies, especially males, towards dead, infected flies producing Entomophthora spores: come hither and be infected is the fungus-modified message! Some of the fungal secondary metabolites must be neurotoxins because changes in insect behaviour are a common feature of fungal infections. In particular, the infected host is frequently driven to climb up vertical surfaces in the final stages of its life: this might mean up climbing up plant stems, rocks, or to the top of walls and windows in domestic pest insects. Being located at altitude is an advantage for spore distribution by the fungus, but it is the dying, infected insect that does the climbing.
Eventually, the fungus emerges through the cuticle and an external mycelium grows over the host and, in appropriate environmental conditions, produces the sporing structures. If circumstances are not suitable, for example dry and/or cold, some fungi form resting structures inside the insect cadaver that produce infective spores when conditions improve.
Toxins from insect pathogenic fungi show promise for control of other pests. Slugs and snails are major crop pests, but the outdoor use of metaldehyde, the main pesticide used to control slugs in crops and gardens, is to be banned across the UK from spring 2020 because it poses an unacceptable risk to birds and mammals. Khoja et al. (2019) investigated the response of representative molluscs to conidia and volatile organic compounds of the insect pathogenic fungus Metarhizium brunneum. They found that conidia of M. brunneum had antifeedant/repellent properties, and aqueous formulations of volatile organics from the fungus, particularly 3-octanone and 1-octen-3-ol, could be sprayed onto plants to kill terrestrial molluscs with no phytotoxic effects. The ephemeral nature of these compounds makes them excellent candidates for development as molluscicides.
Updated October, 2019
