17.2 Culturing fungi
If you are planning to prepare a culture, the first consideration is the nature of the medium. For in vitro cultivations, the medium in which you grow your microorganism must contain all of the elements that the organism contains; Table 2 can help here, by indicating the elemental composition of a typical ascomycete filamentous fungus and a typical bacterium. Table 2 provides information about the type and quantities of nutrients required in a medium; industry does not want to waste nutrients in spent medium and therefore adjusts medium composition accordingly. Heterotrophs use reduced, preformed organic compounds as sources of carbon and energy. There is no naturally occurring organic compound that cannot be used by some microorganisms. Unfortunately, however, many man-made organic compounds (like plastics and pesticides) are degraded slowly or not at all.
During balanced growth an increase in biomass is accompanied by a comparable increase of all other properties of the population, such as protein RNA, and DNA content; in general, the chemical composition of the culture remains constant. The term unbalanced growth describes instances where the relative concentrations of different of macromolecules and other components of the biomass is altered; for example when the nitrogen source has been exhausted but the carbon source is present in excess and the various cellular components are synthesised at unequal rates. Most media are designed so that the carbon and energy source, usually glucose, is the first nutrient to become exhausted.
Table 2. Approximate elemental
composition of Fusarium venenatum and Escherichia coli
(composition is shown as mg g-1 biomass) |
|||||
|---|---|---|---|---|---|
Macro-elements, making up about 96% of the biomass |
Minor and trace elements making up about 4% of the biomass |
||||
Element |
Fusarium venenatum |
Escherichia coli |
Element |
Fusarium venenatum |
Escherichia coli |
Carbon |
447 |
500 |
Minor cations |
||
Oxygen |
Not done |
200 |
Potassium |
20.0 |
10 |
Nitrogen |
83 |
140 |
Sodium |
Not done |
10 |
Hydrogen |
69 |
80 |
Calcium |
0.8 |
5 |
Phosphorus |
16 |
31 |
Magnesium |
1.8 |
5 |
Sulfur |
Not done |
10 |
Iron |
0.05 |
2 |
Trace cations |
|||||
Copper |
0.04 |
3% in total, not separately
measured |
|||
Manganese |
0.12 |
||||
Zinc |
0.28 |
||||
Cobalt, molybdenum, nickel and a
few other metals are important trace elements but were not distinguished
in these analyses. |
|||||
Macroelements, which are those required in the medium in ‘gram per litre’ (g l-1) quantities obviously include carbon, oxygen and hydrogen and also:
- Nitrogen; required for synthesis of amino acids, purines, pyrimidines, etc. Many microorganisms can use the nitrogen in amino acids. Other nitrogen sources include NH4+, NO3-.
- Phosphorus is present in nucleic acids, phospholipids, nucleotides such as ATP etc. Almost all microorganisms use PO42- as a source of phosphorus.
- Sulfur is required for synthesis of the amino acids cysteine and methionine and enzyme cofactors like coenzyme A. Many microorganisms use SO42- as a source of sulfur.
Minor cations, which are required in ‘mg per litre’ (mg l-1) quantities in the medium:
- Calcium concentration in microbial cells is maintained at extremely low levels by highly specific transport processes. Calcium is an important growth regulator. It is easily released from glassware, which can be a source of contamination for calcium-limited medium.
- Iron, (Fe2+ (= ferrous) and Fe3+ (= ferric)), is a constituent of cytochromes, haeme proteins and many other enzymes. Microorganisms need medium concentrations of 0.36 to 1.8 × 10-3 M iron for growth but, under aerobic conditions at pH 7, Fe3+ has a solubility of only 1 x 10-17 M. Although solubility of Fe2+ is increased under acid conditions, and under anaerobic conditions, Fe2+ may attain a concentration of 10-1 M, successful uptake of iron in most circumstances depends on biological iron chelators, called siderophores, or materials added to the medium, such as citric acid or ethylenediamine tetra-acetic acid (EDTA).
- Magnesium is the most abundant intracellular divalent cation. About 90% of intracellular magnesium is bound to ribosomes and polyanionic cell constituents; the remainder constitutes a relatively constant free concentration of 1-4 mM Mg2+. It has a role in macromolecular synthesis and formation of the energy-rich compound ATP. Enzymes requiring magnesium include superoxide dismutase.
- Potassium is required for activity of a number of enzymes and is associated with RNA, but its most significant contribution to fungi is to provide the bulk of the osmotic potential of the hypha.
- Sodium is generally regarded as an essential element but a definitive requirement for growth can rarely be demonstrated, though it is certainly required by marine microorganisms.
Trace cations are those required in µg quantities per litre of medium (µg l-1). They include:
- Cobalt, which is a component of vitamin B12;
- Copper is the prosthetic group of a number of enzymes, including laccases;
- Molybdenum, nickel and zinc are also involved in numerous enzymes in several crucial areas of metabolism.
Where there is a deficiency of a trace clement, such as Fe3+, the specific growth rate is reduced but biomass yield is largely unaffected (Fig. 1).
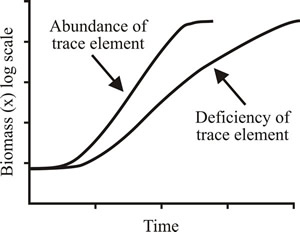 |
Fig. 1. Effects of trace element deficiency on growth in batch culture. Growth rate is reduced but biomass yield is largely unaffected. |
|---|
Table 3 shows recipes for a few standard media and includes preparation notes. During media preparation there are several precautions that are necessary to ensure that the chemistry of the medium does not change when it is sterilised:
- Glucose solutions should be filter sterilised or autoclaved separately from other medium constituents because glucose decomposes (browning caused by caramelisation) if autoclaved in the presence of inorganic salts or organic compounds.
- Ammonium salts should be autoclaved at pH less than 7 to avoid volatilisation of ammonia.
- In contrast, phosphate salts should also be autoclaved separately from other medium components; otherwise insoluble precipitates can form of magnesium ammonium phosphate, magnesium potassium phosphate or magnesium sodium phosphate.
- Iron is a particular problem as without chelating agents all the iron is likely to precipitate. To prevent precipitation of iron and other trace elements, and to control their concentration in the medium, it is generally essential to use a chelating agent and citric acid is often employed for this purpose. Several media recipes use other metal chelating agents, such as EDTA to serve as metal ion buffers (EDTA has greater affinity for Fe3+ and least for Ca2+ and Mg2+). Unfortunately, EDTA may inhibit growth of some microbes and citric acid may be used as a carbon source following exhaustion of the more readily utilised carbon source in the medium. This can complicate growth studies with such media.
Table 3. Composition and
preparation of some widely used culture media |
|---|
Complex media for fungi Malt Extract medium (ME) (constituents per litre):
Liquid media can be solidified with 12 to 20 g agar per litre. Peptones are nutrient extracts obtained by acid or enzyme hydrolysis of natural protein; these extracts are derived from milk proteins (casein), meat, yeasts and plants and provide a source of nitrogen, carbon (as amino acids and peptides) and other nutrients (such as vitamins and minerals) to the cultures. There are numerous peptones and extracts available from commercial suppliers of media (for example: see https://www.sigmaaldrich.com/ and http://www.bd.com/). |
Minimal medium for fungi Vogel's medium (final composition, constituents per litre distilled water; adapted from Vogel, H.L. (1956). A convenient medium for Neurospora (medium N). Microbial Genetics Bulletin, 13: 42.)
Vogel's Medium is conveniently prepared as four solutions which can be sterilised and mixed aseptically for the liquid medium (agar needs to be added for solid medium).
To prepare 200 ml of Vogel's medium mix the following: 40 ml solution C + 4 ml solution A + 56 ml water + 100 ml 3% (w/v) water agar (for semi-solid medium) or use 156 ml water for liquid medium. |
Vogel’s medium is a convenient medium for Neurospora and other ascomycetes; citric acid is the chelating agent (and weak buffer) in this medium and in the absence of an alternative, some fungi can use citrate as a carbon source, which complicates matters. Autoclaving can have a damaging effect on medium composition: a precipitate is formed that contains all the iron and most of the calcium, manganese and zinc; addition of EDTA to the medium prevents this precipitation during autoclaving. There are a great many media recipes. Consult the 'media' entry in 10th edition of Dictionary of the Fungi (Kirk et al., 2008). |
Some components of the medium can be considered to be growth factors in the sense that they must be provided for growth to occur. Although most fungi can synthesise most vitamins, in a few cases specific vitamins are required by some fungi. For example, biotin is a required growth factor of Neurospora crassa and must be added to media, and thiamine is required by Coprinopsis cinerea. We emphasise that these are the characteristics of wild type Neurospora and Coprinopsis; auxotrophic mutants with deficiencies in vitamin biosynthetic pathways can be obtained just as easily as auxotrophs with deficiencies in other pathways. Where the wild type expresses such a biosynthetic deficiency it presumably means that sufficient supplies of the end product are normally encountered in the natural environment of the fungus so the nutritional deficiency causes no adverse selection pressure.
Updated July, 2019
