16.11 Mycoses: the fungus diseases of humans
As we have shown above, there has been an alarming increase in the number of fungal diseases affecting wildlife populations over the past several decades. Although associated primarily with opportunistic infections, fungal diseases of wildlife have caused some of the most important conservation crises in modern times. Studies on the origins of these epidemics are essential to establish how common saprotrophic fungi can gain the ability to cause disease in animals with which they have co-existed for millions of years, and to help us understand how new diseases emerge. This is not only important for wildlife populations, but for ourselves, too. We may be next in line (Fisher et al., 2012, 2016; Fones et al., 2017). You might think we don’t need more fungal pathogens.
‘Fungi cause more than a billion skin infections, more than 100 million mucosal infections, 10 million serious allergies and more than a million deaths each year. Global mortality owing to fungal infections is greater than for malaria and breast cancer and is equivalent to that owing to tuberculosis and HIV...’ (quoted from Gow & Netea, 2016).
The fungus diseases of humans are called mycoses and the majority, perhaps all, are not caused by dedicated pathogens, but rather by fungi common in other situations that take advantage of a particularly beneficial set of environmental conditions or of a host with defences weakened in some way (so-called opportunistic pathogens). There are about 135 fungal pathogens that cause diseases of humans and domestic animals, and only about 60 species of fungi cause disease in humans; of these about 30 cause superficial infections of the skin and about 30 cause subcutaneous, lymphatic or systemic infections (Fig. 10) together with several other species that cause allergic reactions and some commensal fungi that also affect human health and/or disease states (Köhler et al., 2017; El-Jurdi & Ghannoum, 2017).
The fact that there is a relatively short list of mycoses does not mean that fungus diseases of humans are rare. What the human disease fungi lack in diversity, they make up for by being very widespread. To quote a recent survey, which expands on the quotation above:
‘Nearly a billion people are estimated to have skin, nail and hair fungal infections, many 10’s of millions mucosal candidiasis and more than 150 million people have serious fungal diseases, which have a major impact on their lives or are fatal. However, severity ranges from asymptomatic-mild mucocutaneous infections to potentially life-threatening systemic infections. Moreover, mortality associated with fungal disease at >1.6 million is similar to that of tuberculosis and >3-fold more than malaria. …HIV/AIDS pandemic, tuberculosis, chronic obstructive pulmonary disease (COPD), asthma and the increasing incidence of cancers are the major drivers of fungal infections in both developed and developing countries globally. Recent global estimates found 3,000,000 cases of chronic pulmonary aspergillosis, about 223,100 cases of cryptococcal meningitis complicating HIV/AIDs, about 700,000 cases of invasive candidiasis, about 500,000 cases of Pneumocystis jirovecii pneumonia, about 250,000 cases of invasive aspergillosis, about 100,000 cases of disseminated histoplasmosis, over 10,000,000 cases of fungal asthma and about 1,000,000 cases of fungal keratitis occur annually…’ (Bongomin et al., 2017).
Many people (perhaps everybody?) suffers from Athlete’s foot at some time in their life. This is caused by a tropical import called Trichophyton rubrum, but you don’t have to go to the tropics to collect your share because it so likes warm and moist shoes that it is now distributed throughout the temperate climatic zones and is so common that you can be infected very easily. Athlete’s foot is little more than a nuisance though, as it can be successfully treated with over-the-counter remedies. Humans are exceptional among vertebrates in that their living tissue is directly exposed to the outside world; without protective scales, feathers, or fur, our skin is our defence against all the opportunistic fungi surrounding us. A consequence of this exposure to the environment is that we are prone to fungal invasion of our skin, nails and hair. Athlete’s foot is one example, ringworm is another such infection. This introduces another aspect of fungal biology: ‘ringworm’ is a family of mycoses because the cause can belong to one of two closely related genera, Microsporum or Trichophyton. Each fungus is very specific to a specific part of the body. A range of animals can also suffer ringworm diseases of skin and fur, and that range includes farm animals and pets. The fungi spread readily to humans, which introduces another notion, that of a zoonosis, being a disease that can be transmitted from other vertebrate animals to humans.
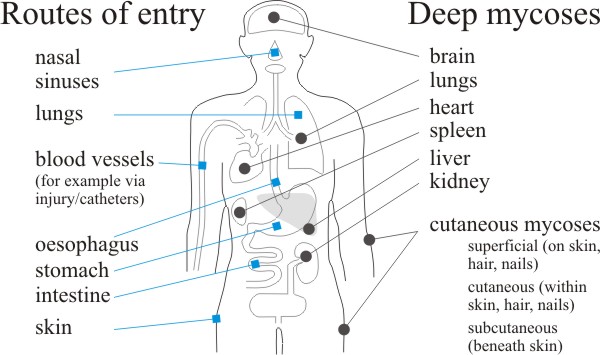 |
| Fig. 10. Routes of entry and distribution of the fungus diseases of humans. Labels at left indicate routes of entry of pathogenic and opportunistic fungi that cause deep and cutaneous mycoses. At right we indicate the principal tissue sites of deep mycoses in comparison with superficial, cutaneous, and subcutaneous mycoses. Based on illustrations produced by Steve Schuenke for the online version of Medical Microbiology, 4th edn, by Samuel Baron (1996), ISBN 978-0963117212 (hardcover), published by the University of Texas Medical Branch at Galveston. |
Another remarkable statistic about human mycoses is that it is now unusual for a woman to go through her reproductive years without at least one significant infection by the yeast Candida albicans. C. albicans is a normal inhabitant of the human mouth, throat, colon, and reproductive organs. Usually it causes no disease but lives commensally in ecological balance with other microorganisms of the digestive system. However, other factors such as diabetes, old age and pregnancy, but also hormonal changes, can cause C. albicans to grow in a manner that cannot be controlled by the body’s defence systems and candidiasis results, with symptoms ranging from the irritating to the life threatening. For most people candidiasis, like other superficial infections, is irritating; the fungus remains in the outer layers of the skin because the body’s immune defence system prevents the fungus penetrating more deeply.
In patients whose immune systems are compromised in some way there is no such defence and the infecting fungus becomes deep-seated, systemic and potentially fatal. This category of patient includes transplant patients, where the immune system is pharmaceutically modulated to control rejection, and people with AIDS (acquired immune deficiency syndrome), where deterioration of the immune system is caused by the decline in CD4+ T cells, the key infection fighters of the immune system, by the HIV (human immunodeficiency virus) retrovirus. Infections of those with weakened immune systems are called opportunistic infections. Candidiasis is the most common HIV-related fungus infection that can affect the entire body, but there are other opportunistic fungi that typically do not cause disease in healthy people but only those with damaged immune systems; these fungi attack when there is an ‘opportunity’ to infect and can cause life-threatening disease. The nature of the protective immune response to fungal invaders, and the balance between immune surveillance, disease progression, host invasion and pathology, and other factors that predispose us to infection must be defined before we can effectively manage human fungal infections (Gow & Netea, 2016; Dambuza et al., 2017; de Hoog et al., 2017).
Several filamentous fungi have emerged as new causes of human infections in recent years. There is some uncertainty as to whether such emerging infectious diseases (EIDs) of humans are caused by newly discovered fungi or are infections caused by fungi that are only now being correctly identified. The truth, as usual, is probably a mixture of all these causes.
Recently developed methods of DNA sequence analysis and mass spectrometry have identified several previously unknown species of Aspergillus in clinical samples. Strictly-speaking, these are not ‘new fungi’ but rather newly recognised species that the classical morphology-based identification of Aspergillus could not distinguish. The other end of the spectrum is illustrated by a case of gastrointestinal basidiobolomycosis causing bowel perforation in a child. In this case the 10-year-old patient presented with inflammatory bowel disease (fever, abdominal pain, vomiting) and was first misdiagnosed as suffering intestinal malignancy or schistosomiasis. The patient’s condition deteriorated despite anti-schistosomal therapy and radical surgery, and only improved following treatment with itraconazole after ‘second opinion’ histopathology diagnosed infection with Basidiobolus ranarum, which was unequivocally identified by PCR. Basidiobolus ranarum more typically causes an unusual fungal skin infection that rarely involves the gastrointestinal tract. Once considered rare, there is an increasing number of reported cases of gastrointestinal infection caused by Basidiobolus species worldwide, in countries such as United States, Thailand, Australia, Iran, Egypt and Saudi Arabia (El-Shabrawi et al., 2011; Roilides, 2016; Shaikh et al., 2016). And even ‘our favourite mushroom’, the Ink Cap Coprinopsis cinereus, can cause disseminated infections in immunocompromised patients, often leading to death. It has been recorded as the cause of endocarditis in 1970 (Spellerya & Maciver, 1971), and an invasive wound infection in a child in 2017 (Correa-Martinez et al., 2018).
Updated July, 2019
