16.2 Microsporidia
Microsporidia comprise a phylum of over 1,400 species of fungal-related parasites that can infect nearly all animal hosts. Microsporidia are the smallest of eukaryotes: they have genomes in the same size range as bacterial genomes; they have unicellular spores; they lack mitochondria, peroxisomes and centrioles, but have several prokaryotic characteristics, such as 70S ribosomes, and fused 5.8S and 28S rRNAs. Nevertheless, they are highly specialised eukaryotic cells, living only as obligate intracellular parasites of other eukaryotes. Most are important pathogens of insects, but they are also responsible for common diseases of crustaceans and fish, and have been found in most other animal groups, including humans (probably transmitted through contaminated food and/or water) (Weiss & Becnel, 2014; Troemel, 2017). Interestingly, a potential malaria control approach has been associated with a vertically transmitted microsporidian symbiont of mosquitoes which has been shown to block Plasmodium transmission (Herren et al., 2020).
Microsporidia have been subjected to the most dramatic taxonomic revisions over the years. They were traditionally thought to be a unique phylum of spore-forming protozoa. Then, on the basis of the earliest electron microscopy studies, they were presumed to be one of the most primitive eukaryotic lineages because of a remarkable absence of ‘standard’ eukaryotic features such as Golgi bodies, peroxisomes, mitochondria and the 9+2 arrangement of microtubules. They were placed with other protists lacking mitochondria in a kingdom called Archezoa, and microsporidia were postulated to be direct descendants of a primitive eukaryote that predated mitochondrial endosymbiosis.
Finally, genome sequencing and the discovery of microsporidian mitosomes, which are highly reduced (in both physical size and biochemical properties) mitochondrial relics, caused the re-classification of microsporidia as fungi. Microsporidia share several characteristics with fungi: nuclear division takes place within an intact nuclear membrane, they have the same mRNA capping mechanism, chitin and trehalose in their spores, and some gene structures present only in fungal opisthokonts (Corradi & Keeling, 2009; Capella-Gutiérrez et al., 2012).
While the fungal nature of microsporidia is now accepted, their exact position in the fungal tree of life is still uncertain. They are now thought to be related to the Chytridiomycota and may be derived from a reduced endoparasitic chytrid (see Section 2.8) (Gill & Fast, 2006). In this case they are now presumed not to be the most primitive eukaryotes, but to be the most reduced and highly specialised fungi. Adaptations to an obligate intracellular lifestyle have modified their cell biology and genome by severe selective reduction of cell structure, metabolism, and gene structure as they separated from the main fungal lineage as a sister clade (see Fig. 10 in Chapter 2; CLICK HERE to view the page).
Microsporidian genomes, although they are always very small, do have multiple linear chromosomes with telomeres, but in other respects they resemble bacteria by having few introns and transposons, generally short intergenic regions, and few duplicated genes. The complement of genes encoded in microsporidian genomes is remarkably similar from one species to another, regardless of genome size, and it is evident that microsporidia have acquired specific genes from unrelated lineages through horizontal gene transfer (see Section 17.15); many of these genes playing a central role in microsporidian evolution (Keeling et al., 2014; Corradi, 2015).
Although their taxonomic classification has evolved through time, understanding to which kingdom microsporidia belong is not an idle academic pastime. Microsporidia cause diseases and it is essential for the clinician to understand that the disease organism is a fungus and that the disease is likely to be controllable by antifungal agents rather than wasting time and resources trying the effects of antibacterials or antiprotozoal drugs.
The first microsporidian to be described, a disease of European silkworms studied in 1857, was shown to be a microscopic parasite that was named Nosema bombycis and was assigned to a new group of organisms called Microsporidia in 1882 (Keeling & Fast, 2002). The microsporidian life cycle ranges from simple to very complex. Reproduction may be asexual, sexual, or both, depending on the species, and some species use intermediate hosts. Some may have several cycles of sporulation and several spore types with different functions including reinfection within the same host. Only the spores are viable outside an animal host.
Microsporidian spore morphology typically includes a thick protective outer wall protein and inner wall of chitin and; but its most obvious morphological feature is the polar filament (Keeling & Fast, 2002). This is specialised for host invasion and provides the microsporidian spore with a unique and highly specialised mode of host infection, where the parasite enters its host through a projectile tube that is expelled at high velocity. The polar filament (or polar tube) is a fine hollow tube, which is attached to a plate at one end of the spore, is then coiled within the mature spore, and terminates near the posterior vacuole at the other end of the spore (Fig. 1) ) (Keeling & Fast, 2002; Vávra & Larsson, 2014).
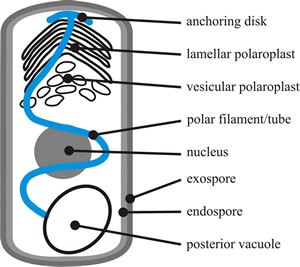 |
| Fig. 1. The major structures of a typical microsporidian spore. The spore cytoplasm is enclosed by a normal plasma membrane with two rigid extracellular walls. The exospore wall has a dense, proteinaceous matrix; the endospore wall, which thins at the apex, is composed of chitin and proteins. The cytoplasm of the spore (the sporoplasm) is the infectious material of microsporidia. It contains either one nucleus or two closely appressed nuclei (= a diplokaryon). The cytoplasm is rich in 70S ribosomes, and is dominated by infection structures: the polaroplast, the polar filament or polar tube, and the posterior vacuole. The membranous polaroplast occupies the anterior part of the spore and is differentiated into the closely stacked membranes of the lamellar polaroplast, and the loosely organised posterior vesicular polaroplast. The polar filament is composed of membrane and glycoprotein layers and ranges from 0.1 to 0.2 µm in diameter and 50 to 500 µm in length, being attached at the apex of the spore via an umbrella-shaped anchoring disk. The polar filament is straight for one third to one half the length of the spore, and the rest is helically coiled in the sporoplasm. The number of coils, their relative arrangement, and angle of helical tilt are conserved and diagnostic for a particular species. The polar filament terminates at the posterior vacuole, though the point of contact has never been observed. Based on illustrations in Keeling & Fast, 2002. |
The spores are ingested by the host and when the spore germinates in the gut, the polar filament everts very rapidly, punctures the membrane of a host cell in the gut wall and effectively injects the content of the spore into the cytoplasm of that cell (Fig. 2). The everted polar filament ranges from 50 to 500 µm in length, which can be up to 100 times the length of the spore. Germination can be complete in less than 2 seconds and the tip of the polar filament can move at more than 100 µm s-1. When the polar tube is fully extended, the pressure within the spore (most likely generated by the posterior vacuole) forces the sporoplasm through the polar tube (Fig. 2F). This also occurs rapidly; the sporoplasm is completely injected into the host within 15 to 500 ms. Following vegetative reproduction in the gut tissues, microsporidia may spread to other tissues.
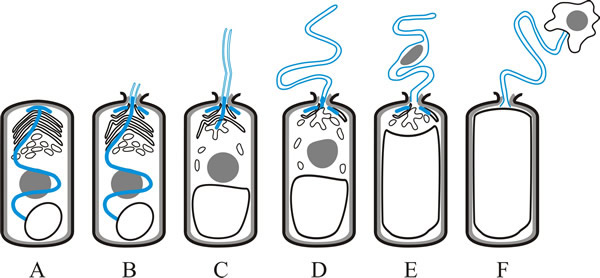 |
| Fig. 2. Germination of a microsporidian spore depends on eversion of the polar filament or tube, and is illustrated in this cartoon strip. A, dormant spore, showing polar filament (blue), nucleus (grey), polaroplast and posterior vacuole (as in Fig. 1). B, polaroplast and posterior vacuole begin to swell and anchoring disk ruptures; polar filament begins to emerge, everting as it does so. C, polar filament continues to evert. D, with the polar tube fully everted, the sporoplasm is forced into and then (E) through the polar tube. F, sporoplasm emerges from the polar tube bound by new membrane. Based on illustrations in Keeling & Fast, 2002. |
If this mode of infection is not remarkable enough, a possibly more remarkable feature of microsporidian biology is the ability of the parasite to stimulate hypertrophic growth (abnormal enlargement) of the invaded cells of the host animal. The organisation of the host cell structure changes completely and the parasite proliferates inside it, but both are morphologically and physiologically integrated to form a separate entity that develops within the host animal but at the expense of the host animal. It seems to be a symbiotic co-existence between the animal cell and its microsporidian parasites to form what is called a xenoparasitic complex rather like a tumour. The term xenoma is now currently used for microsporidian xenoparasitic complex. Microsporidia produce xenomas in oligochaetes, crustaceans, insects and poikilothermic vertebrates, mainly fish (and that includes many commercial fish) (Vergneau-Grosset et al., 2016).
Most microsporidia are specific to one or a few closely related host species. More than half of the described species have been isolated from Lepidoptera and Diptera. Some microsporidia cause acute diseases that result in death of the host, but most are chronic pathogens that reduce longevity and fecundity, prolong larval development, or cause failure to pupate.
Over 1,300 species in 170 genera of microsporidia have been described, and at least 14 species are pathogens of man causing opportunistic infections (generally called microsporidiosis) around the world. Human microsporidiosis occurs mainly, but not exclusively, in severely immunocompromised patients, such as those infected with HIV, and transplant patients.
The most common clinical symptom is diarrhoea caused by general gastro-intestinal infection, but conjunctivitis and other corneal and eye infections occur, as do muscular, respiratory, genitourinary and disseminated infections. Names to look out for as human pathogens are: Brachiola algerae, B. connori, B. vesicularum, Encephalitozoon cuniculi (the most common mammalian microsporidian), E. hellem, E. intestinalis, Enterocytozoon bieneusi Microsporidium ceylonensis, M. africanum, Nosema ocularum, Pleistophora sp., Trachipleistophora hominis, T. anthropophthera, Vittaforma corneae (see the U.S. Department of Health & Human Services, Centers for Disease Control and Prevention web pages at https://www.cdc.gov/dpdx/microsporidiosis/index.html).
Some domestic and wild animals carry microsporidian infections, especially Encephalitozoon cuniculi, E. intestinalis, and Enterocytozoon bieneusi, and are a source of human infections. Cage birds, particularly parrots, parakeets, and budgerigars can also carry infections of Encephalitozoon hellem. Enterocytozoon bieneusi, Nosema sp. and V. corneae have been identified in storm drains and ditches and can contaminate domestic water supplies (but note that the most common waterborne disease caused by faecal contamination of water supplies is cryptosporidiosis caused by Cryptosporidium, which is a protozoan parasite of mammalian intestines).
Loma salmonae causes Microsporidial Gill Disease of Salmon (MGDS) in farmed Pacific salmon, causing severe and extensive inflammation of the gills and consequent respiratory distress and high mortality. It has become an important disease of the Canadian salmon farming industry. The microsporidean parasite Nosema apis infects the epithelial cells of the mid-gut of the honey bee (Apis mellifera) and has spread around the world. It is a serious disease of honey bees in the temperate zones causing severe reduction in productive capacity and contributing to unusually high colony losses in recent years (Mitchell & Rodger, 2011; Charbonneau et al., 2016).
Updated July, 2020
