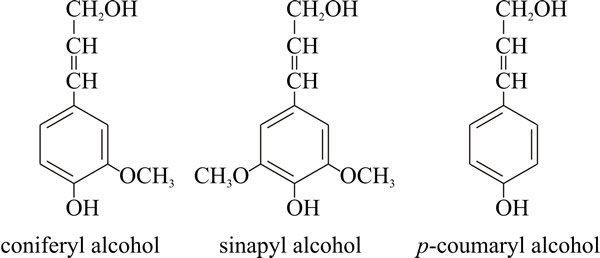
Lignin structureLignins are high-molecular-weight, insoluble plant polymers, which have complex and variable structures. They are composed essentially of many methoxylated derivatives of benzene (phenylpropanoid alcohols, also called monolignols), especially coniferyl, sinapyl and coumaryl alcohols (Fig. 1). The proportions of these three differ between angiosperms and gymnosperms, and between different plants. How lignins are synthesised in the plant is still not settled, despite the many decades spent on its research. A model suggesting that monolignols are assembled at random to produce a complex polymer that is highly cross-linked in three dimensions held sway for many years but is now giving way to the idea that lignins have specific sequences of monolignols that suit the lignin to different functions in the plant cell wall, and that there are only a few native lignin primary structures.
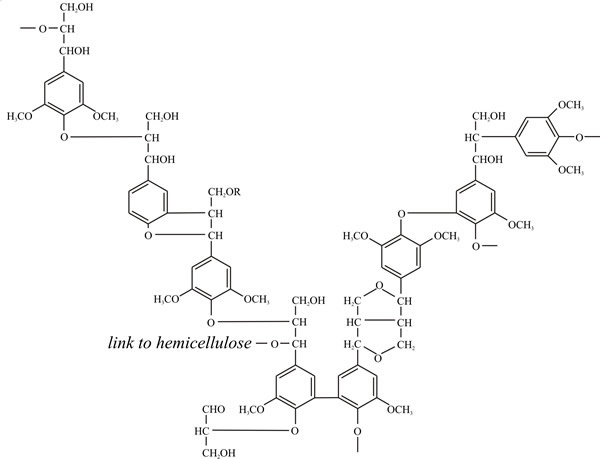
Note what the primary structure of angiosperm lignin (Fig. 2) is telling you about the nature of lignin as a nutritional substrate and the enzymological challenge it poses to any organism setting out to degrade it. Note the way the three phenylpropanoid alcohols are used in the structure; note the predominance of ether linkages (-C-O-C-) and carbon-carbon bonding, and the presence of a few hydroxyls that can take part in cross-links to other polymers (polysaccharides and proteins, for example). Above all, note the predominance of benzene rings. How many foods do you know that are full of benzene rings and how would you metabolise a benzene ring? In your kitchen, benzene rings, especially phenols, are more likely to be found in disinfectants than foods, and that’s the key to the function of lignin. Microorganisms that attempt to degrade lignin produce anti-microbial disinfectants, which makes lignin very resistant to attack. Exactly what the plant needs.
This extreme resistance to microbial degradation is part of the principal function of lignin as it can protect other polymers from attack. We are used to hydrolases cleaving polymers (polysaccharides, proteins, lipids), but the carbon-carbon and ether bonds that join subunits together in lignin must be cleaved by an oxidative process and a range of enzymes are needed for lignin to be degraded. Although ability to digest simple synthetic lignins has been reported for a few bacteria, ability to degrade natural lignins is generally considered to be limited to a very few fungi. The list includes a range of Basidiomycota and Ascomycota, and the fungi involved are generally known as white-rots because the lignin they digest away provides the main pigmentation of wood. Content of this page taken from the new textbook 21st Century Guidebook to Fungi by David Moore, Geoffrey D. Robson & Anthony P.J. Trinci. Published 2011 by Cambridge University Press: ISBN: 9780521186957. URL: ttp://www.cambridge.org/gb/knowledge/isbn/item6026594/?site_locale=en_GB. View Amazon page. |
Except where otherwise indicated, all content copyright © David Moore 2016
All rights of third parties acknowledged