17.11 Chemostats and turbidostats
Even a repeated fed-batch culture is a closed culture system in the sense introduced in Table 1 (above); the essential open culture system is the chemostat. A chemostat consists of a culture:
- into which fresh medium is continuously introduced at a constant rate, and
- the culture volume is kept constant by continuous removal of culture at the same rate, and
- in which the supply of a single nutrient controls growth rate.
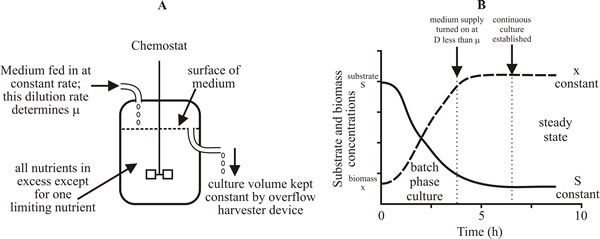
The fermenter is called a chemostat because the growth rate is controlled by the availability of a single component of the medium (the limiting substrate). Important features are that it is the continuous introduction of fresh medium that feeds the limiting substrate to the culture and the dilution rate (that is, the rate of addition of fresh medium) determines the specific growth rate of the culture (Fig. 24) (Matteau et al., 2015).
 |
|---|
| Fig. 24. A basic chemostat. A, Sketch diagram of a continuous culture vessel. B, Record of biomass concentration (x) and substrate (glucose) concentration (S) over the early stages of a cultivation showing the transition from the initial batch phase to the continuous culture phase. |
The main features of a chemostat culture are:
- Volume of the culture remains constant,
- The time required to mix a small volume of medium with the culture should be small, that is, it should approach perfect mixing,
- Environmental conditions can be maintained constant (these include nutrient concentration, pH, temperature, antibiotic concentration),
- Specific growth rate can be varied from just above zero to just below µmax,
- An environmental condition (pH, temperature, oxygen tension, etc.) can be varied whilst maintaining specific growth rate (µ) constant,
- Biomass properties such as macromolecular composition and functional characteristics can be maintained constant,
- Biomass concentration can be set (by altering the concentration of the limiting substrate in inflowing medium) and maintained constant, independently of µ,
- Substrate-limited growth can be maintained indefinitely and this can offer relief of catabolic repression and induction of secondary metabolism at low substrate (glucose) concentration. When chemostat cultures are operated for a very long time the organism evolves (see below); indeed, chemostat cultures can be used to select mutants with particular physiological characteristics (see Table 7).
Dilution rate determines the specific growth rate of the culture:
D = F/V
where D = dilution rate, F = medium flow rate, V = culture volume.
Thus, if F = 1 litre h-1 and V = 5 litres, then D = 0.2 h-1. At steady state µ = D. Thus, µ can be varied in a chemostat from just above 0 to just below µmax. At steady state, the biomass concentration and limiting substrate concentration in the culture remain constant (Fig. 24B). And that link between limiting substrate concentration and biomass concentration is why the rig is called a chemostat.
The biomass concentration in the fermenter vessel at steady state is given by:
bar-x = Y (sr – bar-s)
where bar-x = biomass concentration in the fermenter vessel, bar-s = concentration of growth limiting substrate in the fermenter vessel, Y = yield coefficient for the limiting substrate (Y is about 0.5 for glucose), sr = concentration of growth limiting substrate in the inflowing medium.
The effect of dilution rate on biomass concentration and limiting substrate (glucose) concentration is illustrated in Fig 25, which shows two scenarios in which the concentrations of glucose in the medium entering the chemostat is either 1.0 or 0.2 g l-1.
As the culture approaches µmax (1.0 h-1) biomass is washed out of the vessel and residual glucose concentration increases. The mean residence time of the organism in fermenter vessels is the reciprocal of the dilution rate (that is, R = 1/D, where R = mean residence time of the organism and D = dilution rate).
 |
|---|
| Fig. 25. The effect of dilution rate on biomass concentration and limiting substrate (glucose) concentration. As the culture approaches µmax (1.0 h-1) biomass is washed out of the vessel and residual glucose concentration increases. |
At dilution rates below what is known as the critical dilution rate (Dcrit), steady state can be maintained; at dilution rates above Dcrit steady states cannot be maintained because if D is equal to or greater than µmax a steady state culture cannot be maintained and the culture will washout (more biomass is lost through the overflow than can be replaced by further growth). The kinetics of washout of biomass from a chemostat culture can be used to calculate µmax since:
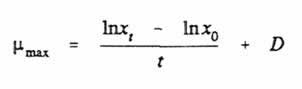
There are two main types of continuous flow cultures, turbidostats and chcmostats (Table 7). Both must be perfectly mixed suspensions of biomass to which the medium is fed at a constant rate and culture is harvested at the same rate. In the chemostat the specific growth rate is controlled externally by the concentration of a single nutrient, the limiting-substrate. In a glucose-limited chemostat, almost all the glucose is utilised by the culture with the result that biomass concentration reaches a constant value which is essentially proportional to the glucose concentration in the inflowing medium (this is the steady state).
Table 7. Comparison between
turbidostat and chemostat continuous flow cultures |
||
|---|---|---|
Parameter |
Turbidostat |
Chemostat |
Method of growth rate control |
Internal |
External |
Growth rate of culture |
At or close to µmax |
From just above zero to just below µmax |
Effect of increasing concentration of all nutrients in the medium reservoir |
Biomass concentration will only be increased if the control settings on the photometer are changed |
Increase in biomass concentration |
Culture volume |
Constant |
Constant |
Environmental conditions |
Constant |
Constant |
Duration of culture |
Indefinite |
Indefinite |
Types of mutants selected by prolonged culture |
µmax mutants and a range of neutral mutants |
µmax mutants at high dilution rates, Ks mutants at low dilution rates, and a range of neutral mutants |
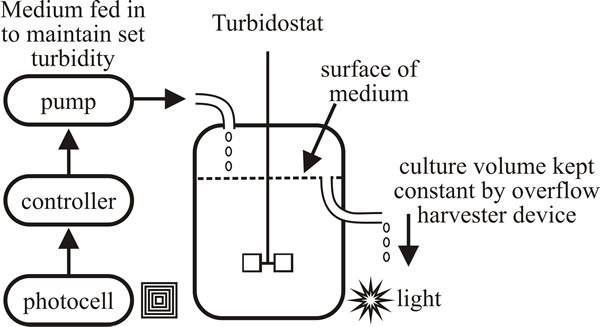
The specific growth rate of a turbidostat culture is at or very close to µmax and is controlled by the rates of internal cellular reactions as they are expressed in the optical density of the culture biomass (that is, the turbidity of the culture). This is done by photometers in which incident light is scattered by the culture and the transmitted light is detected and measured by the photometer; when turbidity (that is, transmitted light detected) reaches a certain level, a medium-pump is switched on to return the turbidity to the required level by feeding medium to the vessel (Fig. 26).
The aim is to hold culture turbidity constant by manipulating the rate at which medium is fed. If the turbidity tends to increase, the feed rate is increased to dilute the turbidity back to its set point. When the turbidity tends to fall, the feed rate is lowered so that growth can restore the turbidity to its set point. The optical surfaces of the detectors most often used to measure turbidity are easily fouled by growth of microbial biofilms, foam or precipitates from the medium and the problems presented by this fouling have not been solved. In practice, a turbidostat operates well for a brief period, but the control of the turbidity eventually becomes unreliable.
In a culture controlled this way, increasing nutrient concentration will affect biomass concentration but not specific growth rate. As shown above, the biomass concentration in the fermenter vessel at steady state is given by bar-x = Y (sr – bar-s).
 |
|---|
| Fig. 26. Schematic of a turbidostat. Photocells measure the light transmitted through the turbid culture; when turbidity (that is, transmitted light detected) reaches a certain level, a medium pump is switched on to return the turbidity to the required level. |
If turbidostats can be maintained for long periods, the system will apply selection pressure for mutants with increased µmax values. Turbidostats have also been used to select mutants with increased resistance to an antibiotic.
Other methods used to monitor biomass and control medium supply include:
- measurement of carbon dioxide in the medium;
- measurement of oxygen in the medium;
- residual nutrient concentration (called a nutristat or
glucose-stat; for Quorn mycoprotein
production, Fusarium venenatum is grown in glucose-stat continuous cultures); - medium pH (called the pH auxostat, which controls addition of alkali to the medium);
- dielectric permittivity (permittivity is of the ability of a material to transmit (‘permit’) an electric field), which is affected by changes in the culture as it grows and is measured with a radio-based sensor that controls addition of medium (such chemostats are called permittistats).
Updated July, 2019
