17.9 Growth as pellets
Fungi that become aggregated or pelleted in submerged culture display more complex growth kinetics. Initial growth is exponential because during the early stages all the mycelial hyphae of the biomass contribute to growth. However, as small pellets enlarge, nutrients have to diffuse through the pellet and mycelium at the centre becomes progressively nutrient limited so that exponential growth becomes limited to the outside of the (spherical) pellet. When large fungal pellets with hollow centres are cut open there is a smell of alcohol, implying that central metabolism has become anaerobic and fermentative. When fungi grow as large pellets the biomass at the pellet centres produce ‘toxic’ products, which diffuse outwards into the culture medium. These compounds eventually inhibit growth of the peripheral biomass.
Pellets begin to form when spores initially swell prior to germination and changes to the electrostatic charge of the spore wall leads to aggregation of spores, which following germination, leads to the formation of a mycelial aggregate and subsequently pellets. This is not a numerically trivial matter; cultures of Aspergillus nidulans inoculated with 2.3 x 106 conidia cm-3 formed only 2.9 x 103 pellets cm-3, a thousand-fold ‘aggregation factor’.
Several factors influence pellet formation. Inoculum size is often critical with high spore concentrations favouring dispersed growth and low concentrations favouring aggregation and pellet formation. Medium composition and impeller agitation of the culture also play a major role in overall morphology in submerged growth and in some case, a low pH has been shown to favour dispersed (that is, non-pelleted) growth.
Growth kinetics of a culture in pelleted form leads to biomass increase that is linear when plotted as the cubed root of biomass over time. This is called cubic growth. Clearly, growth of moulds which form pellets is based on the growth of a population of spheres and the cubic growth relation emerges due to the spherical nature of the pellet and because the volume of a sphere depends on the cube of its radius:
![]()
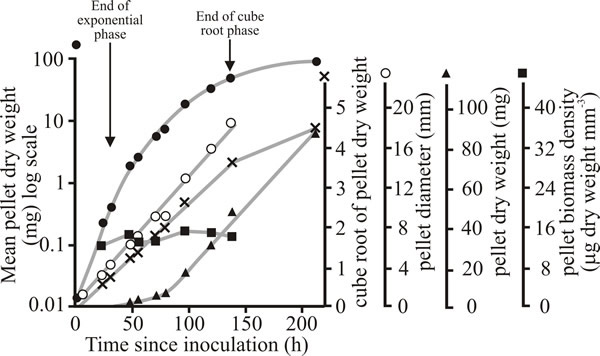
Fig. 18 shows that the increase in dry weight of single pellets of Aspergillus nidulans grown in shake flask culture is better described by cube root kinetics, than by exponential kinetics. This is because the pellet increases in radius at a linear rate determined by the width of a peripheral zone of biomass that is able to continue to grow exponentially; the biomass in regions of the pellet inside this zone is either growing very slowly or has ceased growth.
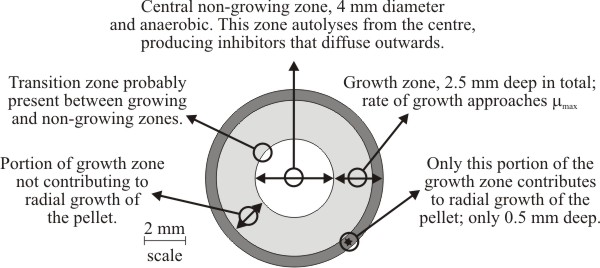
Three zones have been recognised within pellets of Aspergillus nidulans (Fig. 19). The biomass of a pellet is morphologically and biochemically heterogeneous, and although pellets are basically spherical masses of mycelium, the physiology of a fungal pellet is complicated and there is a gradual transition in pellet physiology from its periphery to its centre. The steepness of this transition is influenced by hyphal packing of the biomass (= biomass density), so discrete zonation may be less evident than suggested by Fig. 19.
Generally, speaking however, the outermost zone grows exponentially and supports radial expansion of the pellet. The width of this peripheral zone is determined by the extent to which nutrients (especially oxygen) can be supplied to the biomass in quantities sufficient to support exponential growth. Growth is dependent upon the supply of nutrients to the biomass (by bulk flow and diffusion) and removal by outward diffusion of potentially toxic metabolic excretory products. The innermost zone of the pellet is most poorly supplied with nutrients (especially oxygen) so this zone consists of non-growing biomass. Eventually, the mycelium at the centre of the pellet becomes starved of nutrients, stops growing, enters the stationary phase and finally starts to autolyse (Trinci, 1970) (Fig. 19). As the pellet periphery continues to enlarge this leads to a hollow pellet. Between the non-growing central biomass and the exponentially-growing peripheral zone is the middle transition zone within which increasingly inadequate nutrient (and oxygen) supply causes growth of biomass to proceed so slowly that it does not contribute to radial expansion of the pellet.
 |
|---|
| Fig. 18. Growth of pellets of Aspergillus nidulans grown singly at 25ºC in submerged batch culture (each flask contained 50 ml of medium and was inoculated with a single pellet). Pellet dry weight is shown plotted on logarithmic (closed circles), cube root (crosses) and arithmetic (triangles) axes. The mean diameter of the pellets (open circles) and biomass density (dry weight per cubic millimetre of pellet; squares) are also recorded. |
The growth kinetics of pellets are described by these equations:
![]()
![]()
where, k = a constant, and x = biomass concentration; μ = specific growth rate; w = width of peripheral growth zone of pellet, and t = time.
|
|---|
| Fig. 19. Diagrammatic cross section of a 9 mm diameter pellet of Aspergillus nidulans showing the growth zones recognised within it. Adapted from Trinci, 1970. |
Transport of nutrients into pellets and secondary metabolites out of pellets varies with diffusibility of the metabolite. Oxygen is so poorly soluble in water (see Table 4 and discussion above) that it fails to diffuse into the centre of even small pellets, so the centrally-placed cells consequently die.
Exceptionally, this form of growth can be beneficial from a biotechnology perspective. The classic example of this is in the production of the organic acid citric acid from Aspergillus niger. Citric acid is very widely used as a flavouring agent, preservative and acidity regulator in a range of processed foods and soft drinks. It is also used as a buffering agent in pharmaceuticals and cleaning products, and a metal chelator in soaps and detergents. Originally, citrus fruits, principally lemons, were used as the source of citric acid, a process that was commercialised in Italy in the late 1800’s.
However, some strains of A. niger and other fungi were known to secrete large quantities of citric acid under certain conditions and this was developed in the 1920s by Pfizer in the USA to produce the first dedicated filamentous fungal fermentation. In this fermentation, high yields of citric acid are critically dependent on the culture growing in the form of pellets; citric acid fermentations start with pellets 0.2 - 0.5 mm in diameters which grow to a final diameter of 1 - 2 mm. In addition, efficient conversion of sugar to citrate can only occur if formation of the alternative organic acid, oxalate, is minimised. The key enzyme in the oxalate pathway, oxaloacetate hydrolase, requires the metal manganese and pH values in the range 5-6. Imposition of metal deficiency at very acid pH can completely block oxalate formation, leading to efficient accumulation and secretion of citric acid (Plassard & Fransson, 2009). The medium is often treated chemically to remove trace metals and fermenter vessels are made from stainless steel to avoid contamination.
For the production of most other products, particularly enzymes, high yields are associated with a dispersed culture because this growth morphology maximises the proportion of the biomass which is young, active, and productive. Various techniques have been employed to discourage the formation of pellets. Sometimes this can be achieved by increasing the concentration of the spore inoculum; for example, in Penicillium chrysogenum an inoculum containing fewer than 2-3 x 105 conidia ml-1 medium forms pellets, whereas an inoculum with more than 2-3 x 105 conidia ml-1 medium produces filamentous growth.
As pellet formation is often initiated by electrostatic interactions between spores and sporelings, dispersed growth can be favoured by addition of charged anionic polymers to the culture medium. Effective materials include Carbopol (carboxypolymethylene), Junlon (polyacrylic acid) and Hostacerin (sodium polyacrylate), all of which have been used to discourage pellet formation (Jones et al., 1988). Their mode of action seems to be that they coat the surface of the spores with a layer of uniform negative ionic charge and so prevent spore aggregation by electrostatic repulsion.
Culture morphology also has a major impact on the behaviour of the culture in fermenters. When the mycelium is dispersed, culture viscosity increases as the organism grows due to the interaction of the dispersed, branched, mycelial filaments, and at high biomass levels the culture becomes extremely thick and viscous. This has major implications for the fermentation:
- more energy is required to maintain the speed of stirring of the impellers because of the increased resistance of the culture;
- the rate at which oxygen is transferred to the medium is reduced as the viscosity increases.
This latter is the main reason why oxygen is often limiting in industrial fermentations of filamentous fungi. The overall behaviour of the culture under mechanical stress is known as the rheology of the culture (Fig. 20). Rheology is the study of friction between fluids; it is the apparent viscosity, or ‘resistance to flow’, shown by a complex fluid.
 |
|---|
| Fig. 20. The overall behaviour of the culture under mechanical stress is known as the rheology of the culture. |
Culture rheology is primarily affected by the morphology of the mycelium. When the mycelium is relatively unbranched (high hyphal growth unit; see the sections of Chapter 4 entitled Mycelium growth kinetics and Growth kinetics; CLICK the section titles to view now) the culture viscosity is higher compared to the same concentration of biomass of a highly branched mycelium (low hyphal growth unit). This is because long, sparsely branched hyphae will intertwine and interact more readily than shorter, more highly branched mycelia which behave more as independent entities (Figs 21 and 22).
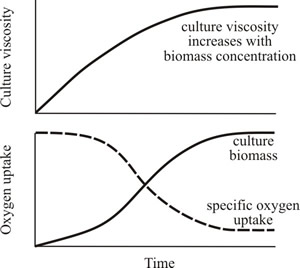 |
Fig. 21. Cultures of filamentous fungi have a non-Newtonian behaviour and viscosity is related to biomass concentration and branching frequency. Top panel shows how culture viscosity increases with biomass concentration. Bottom panel shows how oxygen uptake declines with increasing biomass concentration (because the rate of oxygen transfer from the gaseous to the liquid phase decreases with increase in viscosity). |
|---|
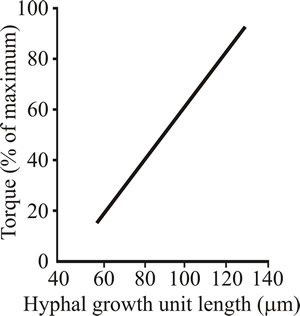 |
Fig. 22. Relationship between hyphal branching (expressed as the hyphal growth unit) and viscosity (expressed as the torque required to rotate an impeller) in Aspergillus oryzae. |
|---|
Updated July, 2019