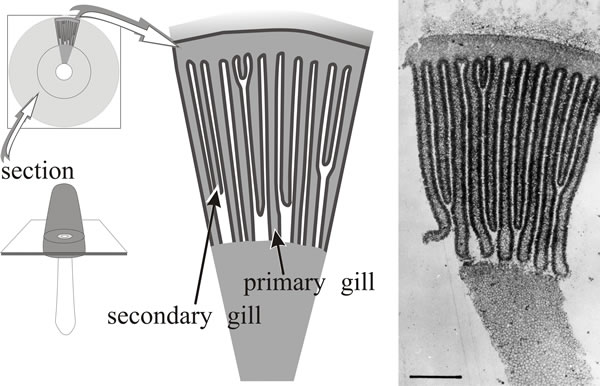
12.6 The Coprinopsis fruit body: making hymenia
In Coprinopsis cinerea, differentiation of the gills (on which the hymenium will be formed) becomes evident in a region corresponding to the boundary between stem and cap tissues before any annular cavity (Fig. 1). The gills arise as vertical ridges of small, closely packed hyphal branches, and as this wave of differentiation moves through the tissue towards the outer surface of the cap it leaves behind two organised plates of columnar cells which constitute the primordial hymenia of adjacent gills separated by a developing gill cavity. An annular cavity is seen only after gills are well formed and it seems to appear as a result of inflation of hyphae in the cap forcing the cap away from the stem.
The central tissues of the first-formed (primary) gills remain intimately connected by interwoven hyphae to the periphery of the stem for a considerable time, becoming freed only in well developed primordia. From this early stage right through to final maturation of the mushroom, developmental changes proceed along two major vectors: from inner edge of the gill (that is, the edge closest to the stem) towards the outer edge (that closest to the exposed surface of the cap) and from the cap margin towards the cap apex (Rosin & Moore, 1985a). These morphogenetic polarities, established during the earliest steps in morphogenesis, are maintained throughout fruit body development.
A mature, fully differentiated fruit body cap of Coprinopsis cinerea consists of a thin layer of cap flesh (the ‘epidermis’ of which is called the pileipellis) bounded exteriorly by the veil cells and with the gills suspended on the inner side. The gills are described as developing parallel to one another and retain this arrangement throughout development; strictly speaking, they are arranged along the radii of the cap, but they are so thin and numerous as to appear parallel when small segments are examined (Fig. 10). The gill surface is the hymenium composed of three highly differentiated cell types: basidia (the cells on which spores are formed, name derived from the Greek basis for ‘step or pedestal’ (of a column) or ‘base’, because they come to support the spores); cystidia (large inflated cells, name derived from the Greek kystis, meaning ‘bladder’); and paraphyses (name derived from the Greek preposition para for ‘beside’ and physis ‘nature’), which are sterile secondary cells, though this description plays down their importance as they eventually form a pavement that provides most of the mechanical structure of the mature gill.
All of these cells arise as hyphal branches from the central layer of the gill, the trama, the constituent cells of which maintain an open and basically hyphal structure. The ‘…ridges of small, closely packed hyphal branches…’ that form the first hymenium of C. cinerea and effectively carve out the main gill plate, are mostly young basidia (often called protobasidia), which will eventually undergo meiosis (Fig. 11) and produce basidiospores, with cystidia scattered amongst them. The latter arise as the terminal compartments of unbranched tramal hyphae (Fig. 11); from the very first, cystidia are much larger cells. They span the gill cavity, connecting together the hymenia of two different gill plates and serve a mechanical function, spreading the tension loads caused by cap inflation through all the gills (discussed below). Only about 8% of the tramal hyphal branches become cystidia; the rest become protobasidia (Horner & Moore, 1987).
Paraphyses originate as branches from the hyphal cells immediately beneath the basidia, and the branches force their way into the basidial layer, inflating as they do so (Fig. 11). About 75% of the paraphyses have inserted by the time meiosis is completed, the rest insert at later stages of development. In contrast, basidia did not increase in number once paraphysis insertion had started (Rosin & Moore, 1985b).
|
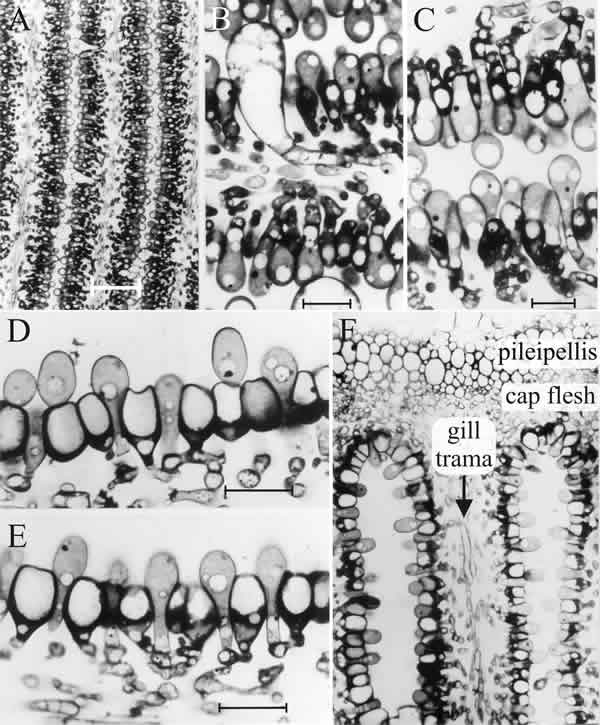
| Fig. 11. The differentiating hymenium of Coprinopsis cinerea in light micrographs of sectioned tissue. A (scale bar: 20 µm), B and C (scale bars: 5 µm) show the hymenium of a primordium 1 to 2 mm tall (similar to that shown in the extreme left photograph of Fig. 12.1). They reveal that at this stage the hymenium consists of a closely packed layer of young basidia, with occasional large, but still young, cystidia. The branches from sub-basidial cells that become paraphyses are evident as more densely-stained hyphal tips, beginning to force their way into the basidial layer. Images D, E (scale bars: 10 µm) and F show views of sections of a later stage in which paraphysis insertion into the hymenium has been accomplished and paraphyses have begun to inflate. Their connection to sub-basidial cells is still very clear. F shows that the pileipellis (‘epidermis’ of the cap) is a layer made up of closely packed cells, like the hymenium. However, the cap flesh is a very open tissue with large intercellular spaces, and the same is true of the gill trama (the gill flesh) in which the hyphal nature of the tissue is clear. Photomicrographs by Isabelle V. Rosin; illustration modified from Moore (1998a). |
Evidence for local control of morphogenesis has been obtained from a comparison of the distributions of cystidia on adjacent hymenia in the Coprinopsis fruit body (Horner & Moore, 1987). The large, inflated, cystidial cells span the gill cavity, impinging upon the opposing hymenium of the neighbouring gill. There are, as a result, certain classes of cystidial relationship (as seen in microscope sections) which are open to numerical analysis. As you can see in the photomicrographs in Fig. 11, cystidia spanning the gill cavity may be ‘distant’, that is having other cells separating them, or ‘adjacent’, with no intervening cells; and, in either case, both cystidia may emerge from the same hymenium (described as cis) or from opposite hymenia (trans) (Fig. 12).
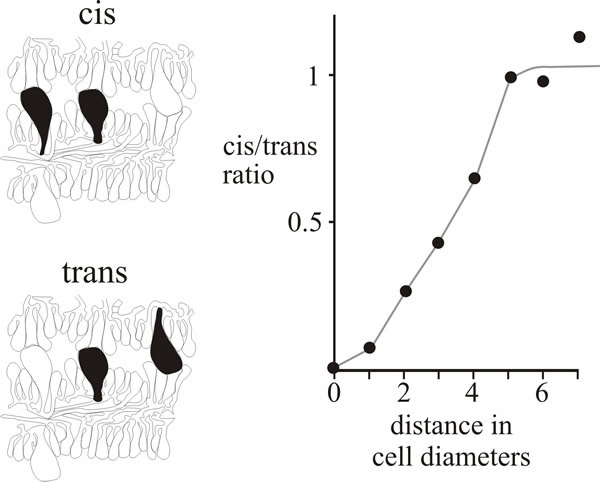 |
| Fig. 12. Cystidium distribution in the Coprinopsis cinerea hymenium. Drawings at left show the categorisation of neighbouring pairs of cystidia in micrographs as either cis (both emerge from the same hymenium) or trans (emerging from opposite hymenia). The graph compares the frequencies of these two types over various distances of separation and shows that closely-spaced cis neighbours are less frequent than closely-spaced trans neighbours, implying some inhibitory influence over the patterning of cystidia emerging from the same hymenium. |
If the distribution of cystidia is entirely randomised then the frequency of adjacent pairs will depend on the population density (which can be measured as a function of the distance between neighbouring cystidia) and there will be an equal number of cis and trans in both the distant and adjacent categories. However, quantitative data from serial sections of a primordium showed a positive inhibition of formation of neighbouring cystidia in the same hymenium (Fig. 12). Evidently, formation of a cystidium actively lowers the probability of another emerging from the same hymenium in the immediate vicinity (Horner & Moore, 1987). The extent of the inhibitory influence extended over a radius of about 30 µm and was strictly limited to the hymenium of origin.
It may be that cystidium formation is activated by the concentration of a component, possibly water vapour, of the atmosphere (assuming this to be gaseous rather than fluid) in the gill cavity immediately above the developing hymenium. The distribution pattern of cystidia would consequently be dependent on interplay between activating and inhibiting factors. Such a patterning process is open to analysis and simulation using the activator-inhibitor model (also called the morphogenetic field model) developed by Meinhardt & Gierer (1974) and Meinhardt, 1976, 1984). This is an elegantly simple model which suggests that morphogenetic pattern results from interaction with just two compounds: an activator which autocatalyses its own synthesis, and an inhibitor which inhibits synthesis of the activator. Both diffuse from the region where they are synthesised; the inhibitor diffusing more rapidly and consequently preventing activator production in the surrounding cells. The model readily accounts for stomatal, cilial, hair and bristle distributions in plants and animals, and a wide variety of patterns can be generated in computer simulations by varying diffusion coefficients, decay rates and other parameters (Meinhardt, 1984). Successful application of this to fungi as well as to plants and animals concentrates attention on the fact that the distribution of stomata on a leaf, bristles on an insect and cystidia on a fungal hymenium have a great deal in common at a fundamental mechanistic level. In other words, these examples are expressions of general rules of pattern formation that will apply similarly to all multicellular systems even though the molecular mechanisms involved are likely to be different.
The descriptions we have just given illustrate a number of developmental control events that indicate exquisite regulation of morphogenesis.
- First, these differentiations are occurring in the gill tissues of the cap while an alternative set of differentiations are happening in the stem tissues, just a few hundred µm distant. The organs of the fruit body are strongly specified regions. We wonder what it is about the control mechanisms that keeps them localised.
- Second, these are fungal tissues so the cellular elements involved are hyphal branches and hyphal compartments (the cells bounded by septa). The branches are formed in such frequency that images such as those in Fig. 11 highlight dramatically the changed growth pattern exhibited by a mycelium during fruit body formation. In vegetative growth on the surface of an agar medium, leading hyphae of this dikaryon produce branches at about 73 µm intervals, but as you can see from Fig. 11 the branching frequency at the hymenium is ten or twenty times greater than that. We wonder what makes them branch so much.
- Third, there are at least four cellular differentiation pathways on display here: undifferentiated hyphae of the trama, basidia, cystidia and paraphyses (we’ll refer to a fifth cell type, cystesia, below); all the cell types mentioned arise as branches from the tramal hyphae, yet despite their relationship as sister branches of the same hyphal system, cells separated only by a dolipore septum clearly follow totally different pathways of differentiation (Fig. 13). We wonder how alternate pathways of differentiation are regulated on the two sides of such a septum.
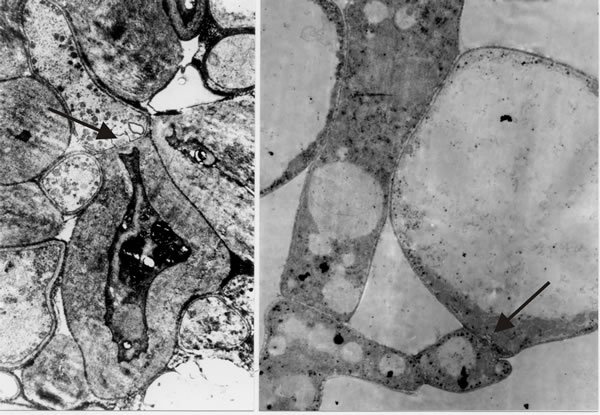 |
| Fig. 13. Transmission electron micrographs of differentiation across the dolipore septum in Coprinopsis cinerea. At left is a section of the medulla of a sclerotium in which thick-walls and thin-walls occur in hyphal compartments either side of a septum (arrow). At right is a section of an immature hymenium in which the enormously inflated paraphysis shares a dolipore septum (arrow) with a cell below which is of more normal hyphal appearance. Images modified from Moore (1998a). |
- Fourth, all of the cells that make up the hymenium are derived from hyphal branches but their tips do not grow continuously, instead their extension is brought under control and they stop extending and differentiate. Further, cessation of extension seems to be coordinated across the entire population of branches so that the surface of the hymenium ends up as a uniform layer. We wonder what mechanism(s) cause all the probasidial branches to stop extending at the same time, and yet allow the (few) cystidial branches to extend across the gill cavity and collide with the opposing hymenium.
- Fifth, the direction of extension of the hymenial branches is also uniform; look at Fig 11A and you’ll see hundreds (in life, hundreds of thousands) of branches from the tramal hyphae all growing upwards to make one hymenium layer, and just a few µm distant from these an equal number of branches from the same tramal hyphae all growing downwards to make the other hymenium layer of the same gill. We wonder what makes the branches in the same layer all turn in the same direction.
- Finally, there is a matter of timing; the first population of branch tips become protobasidia and cystidia, and after they have been specified branches emerge from the first cell (hyphal compartment) beneath each protobasidium and specifically grow into and between the established layers of protobasidia to create the paraphyseal pavement. This process is also coordinated in time and in position across a large population of independent hyphal branches.
The hymenium of Agaricus bisporus adds another interesting example to our catalogue of diversity by using basidia in two different ways: (i) for their primary purpose of sporulation, and (ii) to serve as structural members. Although the Agaricus hymenium is said to contain sterile cells, Allen, Moore & Elliott (1992) found that the overwhelming majority of the constituent cells of the hymenium were protobasidia. Protobasidia with single fusion nuclei were in the majority throughout the life of the fruit body, right through to senescence. Progress through meiosis was slow and at any one time, even in old fruit bodies, only a very small minority of basidia had four daughter nuclei, suggesting that in most protobasidia meiosis was arrested in meiotic prophase I and the arrested protobasidia were kept in that state so they could serve as structural elements in the hymenial layer.
Updated July, 2019