12.4 Ten ways to make a mushroom
There seem to be ten ways to make this mushroom shape (Watling & Moore, 1994) and these are illustrated in the diagrammatic vertical sections shown in Figs 3-9. Sometimes the cap simply grows out of the top of the stem and as the stem increases in height, the cap increases in radius. The hymenium (spore-bearing tissue) forms on the lower surface of the developing cap, but is exposed to the elements from the earliest stages.
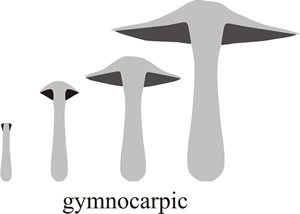 |
Fig. 3. Schematic diagrams showing gymnocarpic development, where the hymenium (dark shading in these vertical section diagrams) simply grows out of the top of the stem, being naked at first appearance and developing to maturity exposed to the elements on the fruit body surface. Modified from Moore (1998a). |
This is called gymnocarpic development; gymnos is the Greek word for ‘naked’ and carpos is Greek for fruit (Fig. 3). For some explanations of Latin and Greek derivations of words used in science see our Resources Box.
Resources Box Latin-Greek Derivatives CLICK HERE to visit a page providing examples of prefixes and suffixes derived from classical languages that are currently used in science. You can download a 5-page PDF of these lists HERE This information was taken from Dr Don Emmeluth’s BioEthics webpages at Armstrong Atlantic State University, Savannah, Georgia, USA at http://www.angelfire.com/de/nestsite/ see also: http://www.demmeluth.net/newindex.html |
Some protection for the young hymenium results when the cap tissues arise shaped like a lid closely applied to the apex of the stem. In this case hymenial tissue arises in an annular cavity formed between the cap and the stem. This cavity eventually expands to completely separate cap and stem but in the early stages the tissue (veil, from the Greek velus, a ‘skin or parchment’) formed between the stem and the closely applied cap protects the hymenium (Fig. 4.). This is called gymnovelangiocarpic development, the word combining gymnos for ‘naked’ (because it becomes naked as development proceeds) and angio which is derived from a Greek word meaning ‘vessel’ or ‘receptacle’ (particularly one containing seed), because the hymenium is enclosed by the veil when first formed.
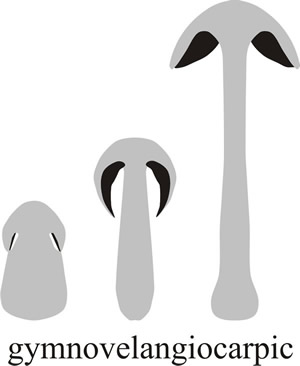 |
Fig. 4. During gymnovelangiocarpic development the hymenium is protected by a much reduced veil, seen only during adolescence, which is formed between the stem and the closely applied cap. From Moore (1998a). |
In other cases the margin or rim of the cap is curled over to protect the hymenium, this is known as pilangiocarpic development (‘hat-receptacle-fruit’ in Greek, see Fig. 5; the word pileus being derived from the Greek pilos which was a close-fitting, brimless hat worn by the ancient Greek sailors). Another variation is that the base of the stem (which is called a stipe, the word deriving from the Latin stipes, meaning a tree trunk or post) may be elaborated into a protective flap (called a volva) that covers the developing hymenium in the early stages (called stipitangiocarpic development, or ‘stipe-receptacle-fruit’ in Greek) (Fig. 6).
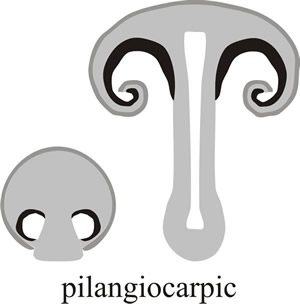 |
Fig. 5. In pilangiocarpic development, the hymenium (black in the diagram) is protected by tissue extending downwards from the margin of the cap to enclose the spore-bearing tissue in the earliest stages and then curling in around the cap margin to protect the youngest hymenium as the fruit body matures. Modified from Moore (1998a). |
|---|
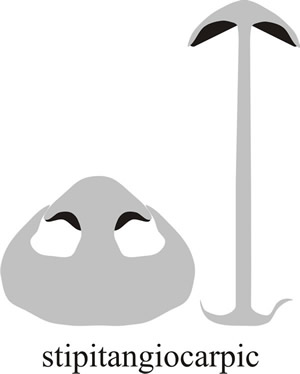 |
Fig. 6. Stipitoangiocarpic development describes an arrangement in which the hymenium is protected by tissue extending upwards from the stem base. This does not enclose the whole primordium and the cap bursts through as it matures, often leaving a cup-shaped remnant of tissue at the base of the stem which is called a volva (which comes from a Latin word meaning ‘a covering’). Modified from Moore (1998a). |
|---|
The most extreme expression of this latter form of protection is bulbangiocarpic development, where the tissue protecting the hymenium is largely derived from the basal bulb of the stem and initially completely encloses the primordium (Fig. 7).
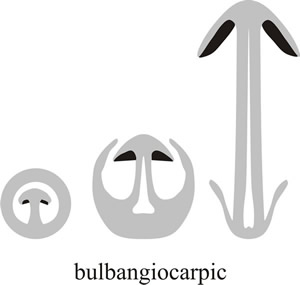 |
Fig. 7. At its most extreme, the volva may extend right over the top of the fruit body and completely enclose it. This is called bulbangiocarpic development, and is the basic structure of the Paddy Straw Mushroom, Volvariella volvacea, and its relatives (see Fig. 27). Modified from Moore (1998a). |
The key feature, and the key function, in these descriptions so far has been protection of the hymenium, and the protective tissues may have different anatomical origins but are most often called veils. This has led to the recognition of four types of velangiocarpic development where one or more special covering tissues (the veils) specifically cover the hymenium (Fig. 8). There may be a single (universal) veil enveloping the primordium in monovelangiocarpic types; or an inner (partial) veil specifically covering the hymenium in addition to the enveloping universal veil in the bivelangiocarpic type. There is also a paravelangiocarpic type in which the veil is reduced and often lost at maturity; and a metavelangiocarpic type, where a union of secondary tissues emerging from the cap and/or stem forms an analogue of the universal veil.
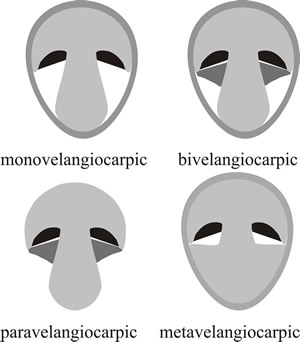 |
Fig. 8. Four types of velangiocarpic development shown as diagrammatic vertical sections through immature fruit bodies. In velangiocarpic fruit bodies the hymenium (shown black in the diagrams) is protected by veil tissue (shaded grey) until the spores mature and it then becomes exposed. Monovelangiocarpic types have a single (universal) veil enveloping the primordium. In the bivelangiocarpic type there is an inner (partial) veil (dark grey) in addition to the enveloping universal veil. In paravelangiocarpic types the veil (dark grey) is reduced and often lost at maturity (the Greek prefix para- meaning ‘closely resembling’ or ‘almost’). In the metavelangiocarpic type a union of secondary tissues emerging from the cap and/or stem forms an analogue of the universal veil (the prefix meta- in this context comes from the Greek and means ‘with’ or ‘among’). Modified from Moore (1998a). |
And finally there are several groups in which the fruit body structure is so modified that it does not develop as a mushroom at all, but may emerge as an even more peculiar fruit body, like a stinkhorn, or a puffball, or a truffle. This is called endocarpic development (from the Greek endon ‘within’ or ‘inside’) and it results in a fruit body in which the mature hymenium is enclosed or covered over. Three patterns of this sort are shown as diagrammatic vertical sections through mature fruit bodies in Fig. 9.
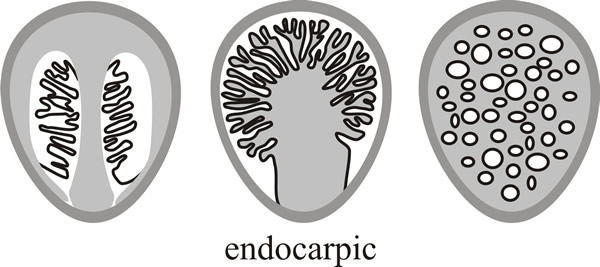 |
| Fig. 9. Endocarpic development produces a fruit body in which the mature hymenium is enclosed or covered over. Three patterns of this gasteromycetous form of fruit body are shown here as diagrammatic vertical sections through mature fruit bodies. Modified from Moore (1998a). |
These fruit bodies, most of which are buried in the soil (hypogeous) or at least at the soil surface, were for many years assigned to a specific taxonomic group, the gasteromycetes (‘stomach fungi’, from the Greek gaster, meaning ‘stomach’ or ‘belly’). However, it became evident that far from defining a natural taxon, the hypogeous fruit body represented a specialisation along many distinct phylogenetic lines. There are some species that produce hypogeous fruit bodies as a departure from their normal epigeous form to cope with adverse environmental conditions (particularly drought and desiccation).
The above discussion is intended to introduce you to the notion that differential growth of different tissues can and does generate a tremendous range of morphological diversity in fungal fruit bodies. This diversity is amplified when you add features like size, pigmentation, symmetry, regular or irregular relationships between the sizes of the component organs, and whether the hymenium is borne on gills, pores or teeth (and whether these are thick or large, thin or small, and/or numerous or few). So if you count up the names assigned to our descriptions above you will find that there are, indeed, ten ways to make a mushroom (CLICK HERE to download a summary diagram of all these as a PDF page); but in numerical terms, that’s just the start of the equation and we hope our descriptions will enable you to think just a little more deeply about the mushrooms in your garden, or those in your mushroom risotto or stir-fry, or alongside your steak.
In and around the British Isles there are 101 known species of mammal, 603 species of birds, 2,951 species of vascular plant, but more than 15,000 species of fungi, a number that rivals the insects (22,500 British species). That’s biodiversity.
From this point we are going to concentrate on cellular details of development and most of the account will deal with an ink cap mushroom now called Coprinopsis cinerea. It is an unfortunate fact of life for those who work with this fungus that it has been misidentified on a number of occasions, so several specific names appear in the literature to which we will refer, including Coprinus cinereus, Coprinus lagopus and Coprinus macrorhizus. They all refer to the same fungus, the different names being the result of misidentifications. Some of the problems of identification are discussed by Moore et al. (1979) who also give a general description of the morphological aspects of fruit body development in the organism. Subsequently, Redhead et al. (2001) used molecular sequence analyses to revise the taxonomy of the whole group that had been called ‘Coprinus’ and subdivided it into Coprinus (in the family Agaricaceae), and Coprinellus, Coprinopsis, and Parasola in the new family Psathyrellaceae. This is how Coprinus cinereus became Coprinopsis cinerea.
Updated July, 2019
