10.5 Breakdown of polysaccharide: chitin
Chitin, in which the repeating unit is the same as that in cellulose except that the hydroxyl group at C-2 is replaced by an acetamido group (Fig. 2), is the second most abundant polymer on Earth as it occurs in the exoskeletons of arthropods and, of course, in fungal cell walls. Polysaccharides which contain amino sugars or their derivatives are called mucopolysaccharides. Chitin is degraded by chitinase, a glucan hydrolase which attacks the β1→4 glycosidic bonds, eventually producing the disaccharide chitobiose which is then converted to the monosaccharide N-acetylglucosamine by chitobiase (Seidl, 2008). Chitinase may also be involved in fungal wall synthesis (see Chapter 6). Several chitinases, which are glycoside hydrolase family enzymes, are produced by fungi and can have different substrate-binding site structures and are obviously specialised for different functions (Zhou et al., 2019). Derivatives of chitin and chitosan have found many commercial uses from medicine to cosmetics and dietary supplements, so fungal chitinases have biotechnological applications, also (Seidl, 2008; Hartl et al., 2012).
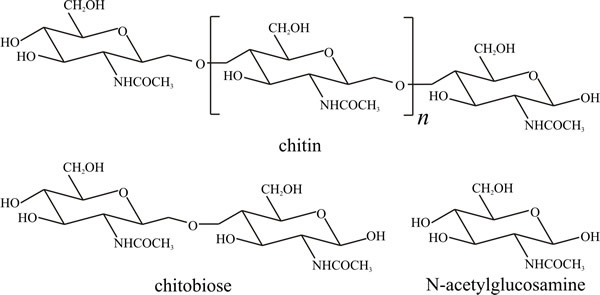 |
Fig. 2. Structural formulae of chitin and its
constituents. Chitin structure is similar to that of cellulose (Fig. 10.1),
except that acetylamino groups (CH3CONH-) replace the hydroxyl groups at carbon
position 2 of the glucose molecule. Approximately 16% of naturally
occurring chitin units are deacetylated. Modified from Moore, 1998. |
