9.4 Aspergillus conidiophores
A great deal of progress has been made in establishing the details of the genetic control of conidiospore formation in Aspergillus nidulans the conidia of which arise on specialised aerial hyphae, the conidiophores. Formation of conidia by surface cultures of A. nidulans occurs after about 16 h hyphal growth. This period of vegetative growth is required to make the cells competent to respond to the induction process, which requires exposure to air and is probably a reaction to cell-surface changes at air-water interfaces. After induction, some mycelial hyphae produce aerial branches which become conidiophore stalks (Fig. 7).
The cell from which the branch emerges is the conidiophore foot cell, which is distinguishable from other vegetative cells by having a brown pigmented secondary wall thickening on the inside of its original wall. The stalk grows apically until it reaches a length of about 100 µm when the apex swells to form the conidiophore vesicle which has a diameter of about 10 µm. A single tier of numerous primary sterigmata, called metulae, then bud from the vesicle and secondary sterigmata, the phialides, bud from the exposed apices of the metulae. The phialides are the stem cells which subsequently undergo repeated asymmetric divisions to form the long chains of enteroblastic conidia which are approximately 3 µm in diameter (Fig. 7).
Classical genetic analysis, by isolation and analysis of mutants, established the basic genetic outline of Aspergillus conidiation. Between 300 and 1,000 gene loci were estimated to be concerned with conidiation by comparing mutation frequencies at loci affecting conidiation with those for other functions (Martinelli & Clutterbuck, 1971). Analysis of mRNA species indicated that approximately 6,000 were expressed in vegetative mycelium and an additional 1,200 were found in cultures which included conidiophores and conidia; 200 of these additional mRNAs being found in the conidia themselves. Only about 2% of mutants of A. nidulans that lacked conidia had defects in stages concerned with conidiophore growth and development, and 85% of conidiation mutants were also defective in vegetative hyphal growth and in attaining competence.
Two genes, in particular, play a key role in conidiophore morphogenesis: these are the ‘bristle’ (brlA) gene which has defects in vesicle and metula formation, and ‘abacus’ (abaA) in which conidia are replaced by beaded lengths of hypha, so it is presumably defective in conidial budding from the phialide and final septation. A third gene, wetA, is defective in an early stage of spore maturation. Conidia of wetA mutants lack pigment and hydrophobicity; they autolyse after a few hours and fail to express a range of spore-specific mRNAs. The wetA gene transcript is lacking in brlA and abaA mutants (i.e. brlA and abaA are epistatic to wetA), and studies of double mutants show that these three genes act in the order: brlA → abaA → wetA.
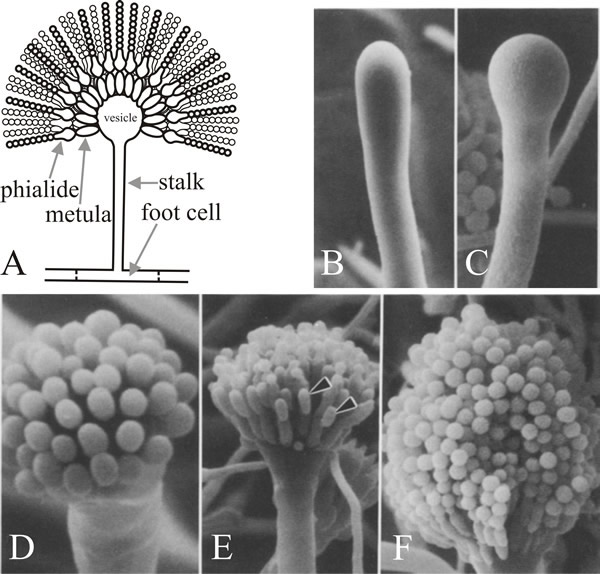 |
Fig. 7. Structure and development of the conidiophore
of Aspergillus nidulans. A, is a diagrammatic
illustration of the structure of the mature conidiophore. B
to F are scanning electron micrographs of various
stages in conidiation. B, Young conidiophore stalk just
prior to vesicle formation. C, Developing vesicle at
the tip of the conidiophore. D, Developing metulae.
E, Developing phialides (arrowheads). F, Tip
of mature conidiophore bearing numerous chains of conidia. Electron
micrographs B to F taken from Mims,
Richardson & Timberlake (1988) with kind permission from Springer
Science+Business Media: © by Springer-Verlag 1988. |
A striking feature of the mutational analysis of conidiophore development in A. nidulans is that mutants of only these three genes cause defects in conidiophore and spore morphogenesis, whereas up to 1,000 other gene loci cause absence of conidiation when mutated. This implies that brlA, abaA and wetA are regulators that integrate the expression of other genes that are needed for conidiation but are not themselves dedicated to it. Many of the Aspergillus conidiation mutants were also defective in sexual reproduction, so another conclusion to be drawn is that there is some economy of usage of morphogenetic genes in different developmental processes. Presumably, different developmental modes employ structural genes that are not uniquely developmental, but function in numerous pathways, having their developmental-specificity bestowed upon them by the regulators to which they respond. This is epitomised in the idea that the key to eukaryote development is in the ability to use relatively few regulatory genes to integrate the activities of many others.
Molecular analysis supports the interpretation that brlA, abaA and wetA are regulators; the brlA sequence encodes a zinc finger protein, which is a sequence-specific DNA-binding transcription activator of developmentally regulated target genes (Fig. 8). However, that’s not the whole story, because the brlA product has different affinities for different target genes. Indeed, the brlA locus consists of overlapping transcription units (Fig. 9), the downstream unit being designated brlAα and the upstream unit brlAβ; between them, their products solve two classic developmental problems, how to respond to a signal and how to maintain that response when the signal has dissipated.
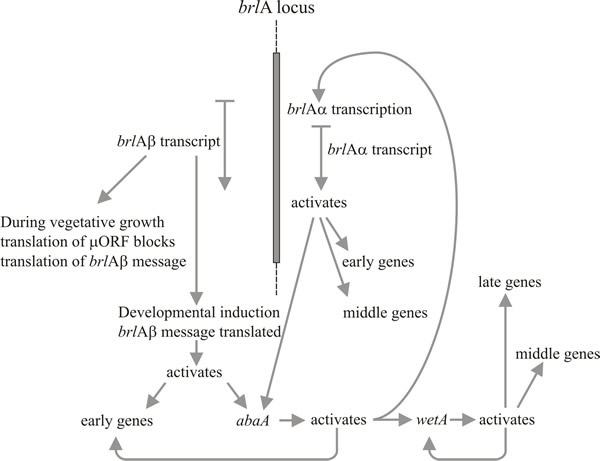 |
Fig. 8. Summary of the genetic regulatory circuit for
conidiophore development in Aspergillus nidulans. |
The two brlA transcription units share the same reading frame for most of their length but brlAβ has an additional 23 amino acid residues at the amino-terminal end of that reading frame, and its transcript also possesses a separate reading frame of 41 amino acid residues (called µORF) near its 5′ terminus. The µORF product represses translation from the downstream (BrlA) reading frame and even though brlAβ transcript can be detected in vegetative hyphae, the BrlA peptide is not produced. The repression caused by µORF is only overcome when the mycelium is competent, which is probably signalled as a nitrogen limitation (a common environmental signal for initiation of sporulation in Ascomycota), which reduces aminoacyl-tRNA pools and disturbs translational regulation by µORF. When the effect of µORF is stalled, the BrlA peptide can be translated from existing transcript.
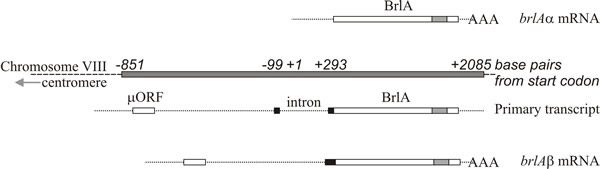 |
Fig. 9. Structure of the brlA locus of Aspergillus
nidulans. The brlAα mRNA is shown at the top, above a shaded box
which represents the BrlA segment of chromosome VIII. The brlAβ primary
transcript and mRNA are shown in the lower half of the figure. The brlAα
sequence is a single exon encoding a Cys2-His2 zinc finger polypeptide
(location of the zinc fingers shown as a shaded box within BrlA). The
brlAβ sequence contains one intron. The polypeptide encoded by brlAβ
contains an additional 23 amino-terminal residues (corresponding
sequence shown as a black box) and the transcript has a short upstream
Open Reading Frame (µORF) which regulates translation of brlAβ. Redrawn
after Timberlake, 1993; and see Lee et al.,
2016. |
The discoverer of this process described the activation of the conidiation pathway as ‘translational triggering’ (Timberlake, 1993), suggesting that the translational trigger is a way of making differentiation sensitive to the nutritional status of the hypha. So the competent hypha is primed to undertake conidiophore development but irreversible activation of the conidiation pathway is prevented by a translational repression that maintains vegetative growth until sporulation conditions are ideal.
Activation of brlA is, therefore, seen as the first step in conidiophore development, and its product in turn activates a panel of conidiation-specific genes among which is the next regulator, abaA. The abaA product is also a DNA-binding transcriptional regulator protein which enhances expression of brlA-induced structural genes. The brlA and abaA genes are reciprocal activators, because abaA also activates brlA. Of course brlA expression must occur before abaA can be expressed, but the consequential abaA-activation of brlA reinforces the latter’s expression and effectively makes progress of the pathway independent of outside events. The abaA product also activates additional structural genes and the final regulatory gene, wetA, which activates spore-specific structural genes. Since brlA and abaA are not expressed in differentiating conidia, wetA is probably involved in inactivating their expression in the spores (and perhaps in the phialide, too; as the current spore nucleus reaches the maturation stage that needs wetA-regulated genes, the phialide nuclei must be ‘turned back’ to the conidium-initiation state to start formation of the next spore). Expression of wetA is initially activated in the phialide by sequential action of brlA and abaA, and it is then autoregulated (the wetA product activates wetA transcription). Positive autoregulation of wetA subsequently maintains its expression after the conidium has been separated (physically and cytologically) from the phialide.
Timberlake (1993) called this mechanism feedback fixation: reciprocal activation, feedback activation and autoregulation of the core regulatory sequences reinforce expression of the whole pathway, making it independent of the external environmental cues which initiated it and enabling the spore to continue maturation even after separation from the phialide. Conidiophore development, like many other morphogenetic processes is naturally divided into sequential steps. This regulatory network shows how translational triggering can relate a morphogenetic pathway to the development of competence on the one hand, and to initiation in response to environmental cue(s) on the other. Subsequent to initiation, feedback fixation results in developmental determination in the classic embryological sense of continuing morphogenesis even when removed from the initiating environment.
We chose deliberately in this section to base our description on research done 50 years or so ago that started with the isolation of a large number of mutations with altered conidiation phenotypes and continued with ‘classic’ studies of gene segregations and gene complementation (Martinelli & Clutterbuck, 1971). Besides showing how our understanding of sporulation has been accomplished, it demonstrates the value of using these ‘old-fashioned’ techniques to investigate such phenomena.
However, we can’t ignore the extensive research which has been completed into the molecular mechanisms underlying growth and development of Aspergillus. These studies have confirmed that the key event in sporulation is activation of the zinc finger transcription factor encoded by brlA, and that abaA and wetA genes are necessary regulators of conidiation. The abaA encoded transcription factor is activated by brlA after differentiation of metulae and during the middle stages of conidiophore development; and the wetA gene, activated by abaA, functions in late phase of conidiation directing the synthesis of crucial cell wall components and transforming the metabolism of the maturing conidium. In Aspergillus nidulans these three genes create the central regulatory pathway, which, with other genes, control conidiation-specific gene expression and the sequence of gene activation involved in the acquisition of developmental competence, conidiophore development, and spore maturation (Lee et al., 2016).
Many aspects of these developmental pathways have been conserved in other aspergilli. Members of the genus Aspergillus are among the most commonly encountered fungi, and all reproduce asexually by forming long chains of conidia. Several species, including Aspergillus oryzae and Aspergillus niger, are used in industry for enzyme production and food processing; while Aspergillus flavus is responsible for food spoilage by producing the most potent known naturally-occurring carcinogens, the aflatoxins. Another species of concern is the opportunistic human pathogen Aspergillus fumigatus, which produces a massive number of small hydrophobic conidia as its primary means of dispersal and has become a widespread airborne fungal pathogen in developed countries. In immunocompromised patients, A. fumigatus causes an invasive aspergillosis that has a high mortality rate. The BrlA-AbaA-WetA developmental signalling pathway has been conserved in conidiation of these Aspergillus species (Yu, 2010; Tao & Yu, 2011; Krijgsheld et al., 2013). Further, proteomic, transcriptomic and metabolomics studies have provided a detailed picture of the dynamic changes that occur in many thousands of genes, transcripts, enzymes and metabolic reactions during formation, maturation, dormancy and germination of Aspergillus conidia (van Leeuwen et al., 2013; Novodvorska et al., 2016; Teertstra et al., 2017), and targeted metabolomics analysis shows promise as a supporting taxonomic tool for various genera of Ascomycota (Ząbek et al., 2017).
Updated June, 2021
