15.6 Nematode-trapping fungi
Fungi are pathogenic, parasitic or symbiotic with a range of different animals, but their relationship with soil nematodes goes a step beyond parasitism and into predation. There are about 700 species of taxonomically diverse fungi that are be able to attack living nematodes (eelworms), which are active animals about 0.1 to 1.0 mm long. Among these nematophagous fungi, only a few species are obligate parasites of nematodes; the majority are facultative saprotrophs. Nematophagous fungi fall into four general groups: (i) fungi that use specialised structures that trap and then invade eelworms; (ii) fungi that invade eelworms after first immobilising them with toxins; (iii) obligate endoparasitic fungi that invade eelworms following spore germination; (iv) opportunistic saprotrophic fungi that colonise nematode eggs, females, or cysts. Nematophagous fungi are natural enemies of nematodes in soil ecosystems and have potential as biocontrol agents against plant- and animal-parasitic nematodes (Jiang et al., 2017; Zhang et al., 2020).
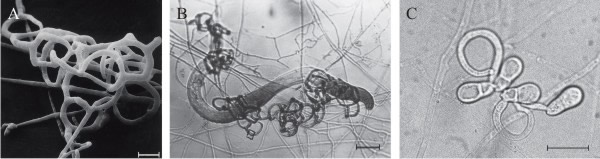
Over 200 species of fungi (zygomycetes, Basidiomycota, and Ascomycota) catch free-living nematodes in the soil using traps produced by the fungal mycelium that adhere to the worm, then penetrate, kill, and digest the tissue of the nematode. The most widespread predatory fungi are in the family of Orbiliaceae (Ascomycota). Five kinds of trapping device have been recognised (Fig. 6); the first four of those listed below capture nematodes using an adhesive layer covering part or the entire surface of the hyphal structure (Yang et al. 2007; Su et al., 2017):
- Adhesive network (AN), the most widely distributed trap, is formed by lateral branches from a vegetative hypha, looping around to fuse with the parent hypha, developing a network of loops with an aperture diameter of about 20 µm (Fig. 5A); this is a three-dimensional network (see Fig. 6A) which entangles the prey (Fig. 6B) and can be formed by germinating conidia (Fig. 6C).
- Adhesive knob (AK) is a morphologically distinct inflated cell that is either a short (‘sessile’) or long (‘stalked’) hyphal branch, which are usually closely spaced along the hypha (Fig. 5B).
- Nonconstricting rings (NCR) always occur alongside AK, and are produced when lateral branches from a vegetative hypha loop and inflate, forming a three-celled ring on a supporting stalk (Fig. 5B).
- Adhesive column (AC) is a short erect hyphal branch consisting of a few swollen cells (Fig. 5C).
- Constricting ring (CR), is also a looped hyphal branch of (usually) three cells, but it is the most sophisticated trapping device (Fig. 5D) and captures prey actively. When a nematode enters a constricting ring the three ring cells are triggered to swell inwards within 1 to 2 seconds and firmly lasso the victim; the cells inflate to maximum size, which is an approximate threefold increase in cell volume, within 0.1 second, with the swelling of the ring cells being strictly inward (Fig. 7).
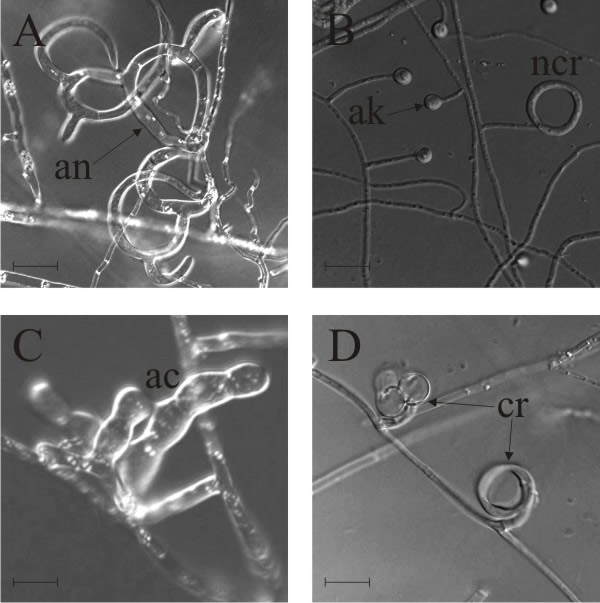 |
| Fig. 5. Natural nematode-trapping devices. A, adhesive network (an), the most widely distributed trap. B, adhesive knob (ak) with nonconstricting rings ncr. C, adhesive column (ac) is a short erect branch consisting of a few swollen cells produced on a hypha. D, constricting ring (cr), the most sophisticated trapping device, captures prey actively; when a nematode enters a constricting ring, the three ring cells are triggered to swell rapidly inwards and firmly lasso the victim within 1 to 2 second. The ring at upper left in panel D has been triggered, that at bottom right is an unsprung trap. Scale bars = 10 μm. Modified from Yang et al., 2007 using images kindly supplied by Prof. Xingzhong Liu and Dr Ence Yang, Institute of Microbiology, Beijing, China. Original images © (2007) National Academy of Sciences, USA. |
If you've not already done so, we recommend that you check out the Life in the soil movies showing soil nematodes and nematode-trapping fungi; hyperlinks are in the following Resources Box.
Resources Box Life in the soil Thomas E. Loynachan, Emeritus Professor of Agronomy and Microbiology at Iowa State University, created a set of 16 short digital videos showing the scope of life in the soil. CLICK HERE to visit a page providing access to these. Life in the soil on YouTube Deep Down & Dirty: the Science of Soil. A close-up of creatures living beneath the soil, made by the British Broadcasting Corporation: https://www.youtube.com/watch?v=gYXoXiQ3vC0 The Living Soil Beneath Our Feet. Made by the California Academy of Sciences: https://www.youtube.com/watch?v=MlREaT9hFCw |
Phylogenetic analysis suggests that the trapping structures fall into two lineages: one based on constricting rings and the other using adhesive traps. Only the traps, not the rest of the mycelium, have adhesive layers, and traps have thicker cell walls than vegetative hyphae and their cells contain electron dense bodies. There is a recognition event between lectins (sugar-binding proteins) in the cell wall of the fungus and one or more carbohydrates on the surface of the nematode; among which are the ascarosides. These are lipophilic glycosides of the dideoxy sugar ascarylose, which serve essential functions in regulating nematode development and behaviour, so they are highly specific to the desired prey of the fungus. Comparative phylogenomic reconstruction of nematode-trapping fungi suggested an evolutionary trend of trapping device simplification in morphology, which was accompanied by expansion of gene families encoding adhesion proteins and their increasing adhesiveness on trap surfaces. Gene expression analysis revealed a consistent up-regulation of the adhesion genes during the fungal lifestyle transition from saprotrophic to nematophagous stages (Ji et al., 2020).
Nematophagous fungi, natural predators of soil-dwelling nematodes, can detect and respond to their prey’s own ascaroside pheromones (Hsueh et al., 2012). An interesting addendum to this story is that some bacteria can mobilise nematode-trapping fungi to kill nematodes. In their soil habitat, bacteria are consumed by bacterivorous nematodes; however, some of these bacteria release urea, which triggers a lifestyle switch in the fungus Arthrobotrys oligospora from saprotrophic to its nematode-predatory, nematode trapping, form; it seems to be ammonia that the fungus produces from the urea that functions as the signal to form traps. This bacterial defensive mechanism significantly promotes the elimination of nematodes by A. oligospora (Wang et al., 2014).
Recognition of the prey results in reorganisation of the adhesive surface polymer on the fungus and adhesion of the nematode to the fungus. It also triggers the growth of hyphal branches into the nematode to initiate its digestion. Interaction between predator (e.g. Arthrobotrys oligospora) and prey (nematode) shows no species specificity. Hyphae penetrate the nematode within 1 hour of capture. These hyphae digest the nematode (Li et al., 2005; Yang et al. 2007; Su et al., 2017).
The capture organs in some predatory nematode-destroying fungi are constitutive, others are inducible. Formation of traps by Arthrobotrys oligospora is induced by presence of nematodes or peptides secreted by them; in the absence of nematodes the fungus grows saprotrophically. It is thought that the nematode-trapping habit is a way that saprotrophic soil fungi compensate for the poor nitrogen content of their substrates. Trap formation and nematophagous activity of Arthrobotrys oligospora were observed in vitro only where conidia were inoculated on nutrient poor water agar, low-nitrogen medium or a medium containing no amino-acids or vitamins.
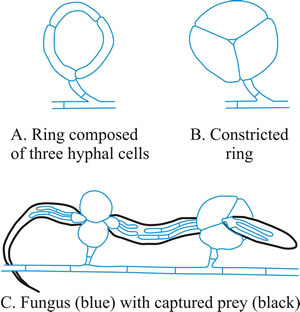 |
Fig. 7. Constricting rings of Drechslerella snap shut. When a nematode enters a constricting ring the three ring cells (A) are triggered to swell inwards within 1 to 2 seconds and firmly lasso the victim; the cells inflate to maximum size, which is an approximate threefold increase in cell volume, within 0.1 second, with the swelling of the ring cells being strictly inward (B). The constricted rings clamp the prey firmly (C). Think, for a moment, what that description means in terms of (a) the sensory and signal transduction system that detects the presence of the nematode, (b) the reaction system that generates a burst of metabolic activity to create the osmotic potential and transport the water to inflate the cells and the localised modification of cell wall architecture that directs the morphological expansion to the interior of the loop. |
Trapping devices remain inducible after many years of culture as saprotrophs on artificial media, demonstrating that these highly differentiated trapping structures remain crucial to the survival and virulence of their producer.
Arthrobotrys is characterised by adhesive networks and unstalked adhesive knobs that grow out to form networks; Dactylellina by stalked adhesive knobs and non-constricting rings, and unstalked adhesive knobs that grow out to form loops adhesive knobs; and Drechslerella by constricting-rings.
Predatory fungi appear to have been derived from nonpredatory members of the Orbiliaceae (Ascomycota), although this is a lifestyle that has appeared several times and in different fungal phyla. For example, the oyster mushroom (Pleurotus ostreatus), which is in the Basidiomycota, is a nematode-trapping fungus that uses adhesive hyphal branches. We recently received this e-mail from Mr John L. Taylor of the North West Fungus Group:
'While I have read about Pleurotus species being able to capture nematode worms, I imagined this skill to be limited to hyphae within the woody host material. However I needed to check a mis-identified Oyster Mushroom, P. ostreatus, so sectioned across cap and 5 gills, finding 15 dead nematodes curled in 2 spaces in the cap tissue. One easily visible had a single cup-shaped hyphal attachment (said to present a nematode toxin), and hyphal growth branching into the nematode from the point of attachment, suggesting ingestion. While this mushroom was structurally sound, it was visually past its edible condition, so those who eat fresh Oyster Mushroom should not be deterred!'
In the orbiliaceous fungi the adhesive knob is considered to be the ancestral trapping device from which constricting rings and networks were derived via two pathways:
- one in which adhesive knobs developed first into adhesive two-dimensional networks, then three-dimensional networks;
- a second pathway in which adhesive was lost and nonconstricting rings developed inflatable cells to form constricting rings (Li et al., 2005; Yang et al. 2007; Su et al., 2017 ).
Nematode-trapping fungi have been found fossilised in amber
dated at 100 million years old. The fossil fungi used hyphal rings as trapping
devices and are preserved together with their nematode prey. Evidently the
predaceous habit of these fungi was well represented in the Cretaceous (the age
of the dinosaurs) (Schmidt, Dorfelt & Perrichot, 2007).
Soil nematodes are very abundant in all soils (commonly millions per square
metre) and species diverse (commonly more than 30 taxa). They feed on a wide
range of soil organisms within the rhizosphere of agricultural crops and several
eelworms are parasitic. Some are important pests of crop plants and farm animals
and nematode-trapping fungi may have use in biological control
(Yeates & Bongers, 1999; Moosavi & Zare, 2012; Moura & Franzener, 2017).
For example, the nematode-trapping fungus Nematophthora gynophila can be used to control the cereal nematode, Heterodera avenae, which feeds on the host roots damaging the roots and reducing water uptake. Nematodes that are gastrointestinal parasites of farm animals can be controlled with the nematode-trapping Duddingtonia flagrans. The resting spores (chlamydospores) can be included in animal feed and survive passage through the animal. Subsequently, the fungus grows in the animal dung where it traps and destroys the parasitic nematode, so reducing pasture infectivity and the worm burden of grazing animals, especially young cattle, sheep and goats. Three months treatment can reduce the worm burden by 90% (Graminha et al., 2005; Larsen, 2006).
Another interesting example is that Trichoderma spp. can improve plant defences against plant pathogenic nematodes. Trichoderma harzianum has been shown to induce resistance to root-knot nematodes by increasing secondary metabolite synthesis and defence-related enzyme activities in the host plant, Solanum lycopersicum (tomato). Root colonisation by T. harzianum significantly upregulated the expression of several plant genes resulting in increased activity of a panel of plant enzymes leading to heightened concentrations of phenols, flavonoids, lignin, and cellulose, and decreased levels of H2O2, malondialdehyde and electrolyte leakage (Yan et al., 2021).
Updated May, 2021