12.7 Coprinopsis and Volvariella making gills (not forgetting how polypores make tubes)
There is a fifth cell type in the hymenium of C. cinerea and its origin illustrates yet another phenomenon found in other organisms. At early stages in growth of the cystidium across the gill cavity the cell(s) with which the cystidium will come into contact in the opposing hymenium are indistinguishable from their fellow protobasidia. However, when the cystidium comes firmly into contact with the opposing hymenium, the hymenial cells with which it collides develop a distinct granular and vacuolated cytoplasm. This suggests a contact stimulus aborting continuation of basidial differentiation in those cells and setting in train an alternative pathway of differentiation leading to an adhesive cell type (called the cystesium, a name derived from ‘cystidium’ plus the Latin root of ‘adhere’; Horner & Moore, 1987) (Fig. 14). The cystidium-cystesium adhesion is very strong and, indeed, is based on joint wall synthesis (shown in Fig. 14C).
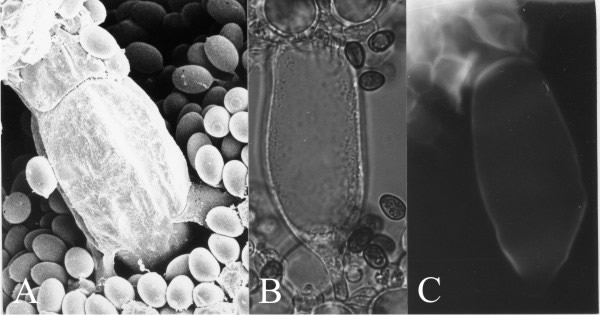 |
| Fig. 14. Cystidia and the adhesive cystesia of Coprinopsis cinerea. A panel of three images all showing a cystidium spanning the gill space and fused with cystesia in the opposite hymenium. A, is a critical-point-dried scanning electron microscope specimen showing the junction line between cystidium (below) and cystesium (above). B, is a bright field light micrograph of a cystidium and its cystesia (above), and C shows the same cell under UV-excitation of the fluorescence of calcofluor white, revealing newly-synthesised chitin in the walls by which the cells adhere. Thus, a cystidium spanning between two hymenia has its connections to both hymenia reinforced. Scale bar = 20 µm. Photomicrographs by Prof. S.-W. Chiu, Chinese University of Hong Kong. Illustration modified from Moore (1998a). |
An interesting description of the consequence of the strength of this joint wall synthesis in ‘Coprinus atramentarius’ is recorded by Buller (1910):
“...When one succeeds in forcibly separating parts of two neighbouring gills, one finds that although the cystidia have mostly separated from one of the gills at their apical ends, and have thus remained attached to the other gill at their basal ends...., not infrequently the reverse happens; the cystidia ... have broken away at their basal ends and have remained attached at their apical ends...”.
Such graphic descriptions indicate that it’s been known for a very long time that adhesion between the cystidium and the cystesium can be so strong as to exceed the breaking strain of the subhymenial hyphal branching system causing cells to be torn out of their hymenium of origin. What could be the function of such a singularly strong adhesion? The answer to this question starts with the observation that when first formed the primary gills of Coprinopsis cinerea appear as convoluted, folded plates (Fig. 15).
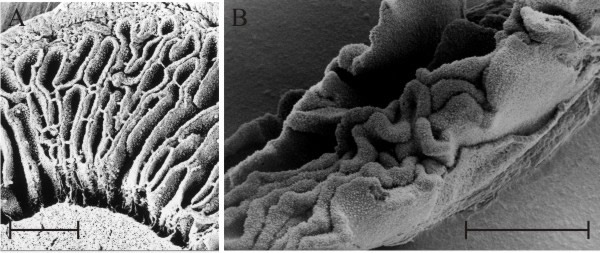 |
| Fig. 15. ‘Embryonic gills’ in both Coprinopsis cinerea (A) and Volvariella bombycina (B) are clearly seen to be convoluted in these scanning electron microscope preparations. The scale bars = 0.5 mm so these are pieces of tissue from very small fruit body primordia, just a few mm in overall size from organisms that produce mature fruit bodies several cm in size. Photomicrographs by Prof. S.-W. Chiu, Chinese University of Hong Kong. Illustration modified from Moore (1998a). |
For such convoluted gills of Coprinopsis cinerea to end up ‘parallel’, it is envisaged that as the cap expands the gills are tensioned into the regular parallel arrangement (shown in Fig. 10). The process is accomplished through the anchorages provided by the connection of primary gills to the stem and by cystidium-cystesium pairs acting as tension elements, interconnecting gill plates into a fully tensioned structure around the stem as cap expansion pulls the gills into shape. The tension is a geometrical consequence of the way the fruit body matures. The primordial cap in Coprinopsis encloses the top of the stem (diagrammed in Fig. 10) with the gills arranged as vertical plates radially around the stem. Simple measurements show that as typical fruit body primordia of C. cinerea grow from one to 34 mm in height, the circumference of the stem increases by a factor of 9 while the outer circumference of the cap increases by a factor of 15. As the primary gills are connected to both of these surfaces they will inevitably be stretched by the outer surface being increased more than the inner, and the regular distribution of strongly adhering cystidium-cystesium pairs will act as tension ties communicating and equalising these tension forces through all of the gills.
Fig. 15B also shows that embryonic gills of Volvariella are convoluted in very young primordia but this species is quite different from the coprinoid-type (compare Fig. 7 with the diagram in Fig. 10). The overall developmental programme for all agaric gills (using the word ‘agaric’ in the widest sense to mean any ‘mushroom’ with gills) seems to be that primary gills arise as ridges on the lower surface of the cap, projecting towards the stem, and secondary and tertiary gills are added whenever and wherever space becomes available. Generation of additional gills occurs by bifurcation of an existing gill either from one side or at the free edge, or by folding of the hymenium layer near or at the roots of existing gills. Application of these ‘developmental rules’ (particularly the ‘whenever and wherever space becomes available’ rule) inevitably gives rise to the sinuous (= labyrinthiform) gill pattern illustrated in Fig. 15B as a normal transitional stage to the regularly radial pattern so often seen in mature agaric mushrooms.
Transformation of the convoluted gills into regularly radial ones is probably accomplished by cell inflation in the gill trama of Volvariella. The mature hymenium of Volvariella (Fig. 16) is a layer of tightly appressed cells, and the trama of the broken gill shown in Fig. 16 is full and crowded with greatly inflated cells. Reijnders (1963) and Reijnders & Moore (1985) emphasised the role played by cell inflation in driving developmental events in fungal structures, so it doesn’t take much to suggest that inflation and growth of tramal cells in gills enclosed by the hymenial 'epidermis' will generate compression forces which will effectively inflate, and so stretch, the embryonic gills to form the regularly radial pattern of the mature cap. Thus, in this case the gill is inflated into shape and, in engineering terms, the mature gill is a stretched-skin construction which owes its structural strength to the combination of compression in the trama and tension in the hymenium (Chiu & Moore, 1990b).
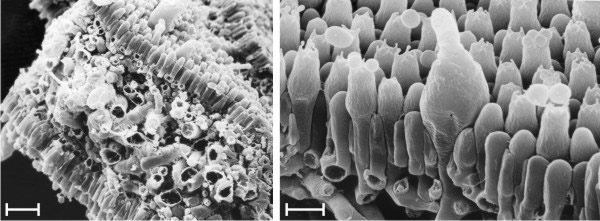 |
| Fig. 16. The mature gill of Volvariella bombycina in scanning electron microscope images. Left is a view of the exposed trama of a gill broken whilst frozen (scale bar = 20 µm), and the right hand image is a magnified view of the hymenium (scale bar = 5 µm). Note that the hymenial cells are closely packed and interlocked to form a coherent layer, like the tightened skin of a balloon, stretched over a gill trama which contains a mass of greatly inflated cells. The gill is inflated into shape and, in engineering terms, is a stretched-skin construction which owes its structural strength to the combination of compression in the trama and tension in the hymenium. Photomicrographs by Prof. S.-W. Chiu, Chinese University of Hong Kong. Illustration modified from Moore (1998a). |
The direction of gill development has been a matter of controversy since the start of the twentieth century. The crucial question is whether gills generally extend by growth at their edge or at their root (or both, or neither). This is not an esoteric point but an important question about which region is growing and therefore the location at which developmental control is exercised. The question can be easily answered for conventional agaric fungi by placing ink marks at measured intervals on immature fruit bodies and observing what happens to them as the fruit bodies mature (Fig. 17). In experiments done by painting water-based ink or water-colour paint onto the live tissue with minimum disturbance to the young fruit body of Volvariella (Chiu & Moore, 1990b), ink marks placed near the cap apex remained close together whereas marks originally close together in the mid regions of the outer surface of the cap became widely separated (and were made more diffuse) by subsequent growth. Also, ink marks placed on the cap margin and those placed on the free edges of the gills remained at the margin or the free edge. These observations show that:
- the greatest contribution to cap expansion occurred in an annulus (that is, a ring) some way in from the margin and not extending to the apex;
- the free edges of the gills remained intact and were not replaced;
- the margin of the cap was similarly not replaced though its circumference increased.
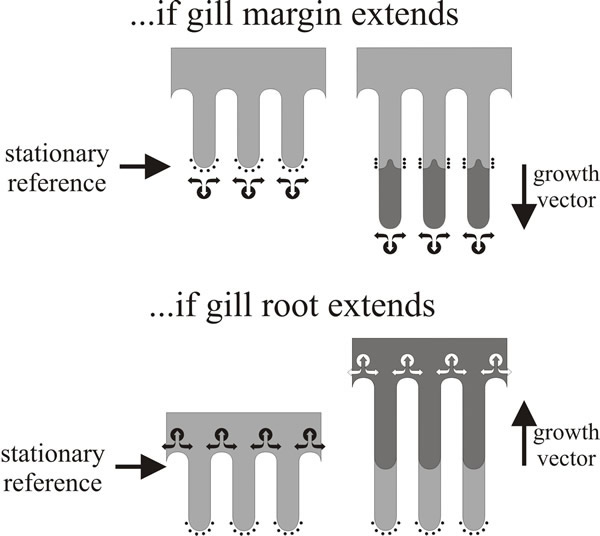 |
| Fig. 17. Gill formation in Volvariella (Chiu & Moore, 1990b). Diagrams of marking experiments done by placing ink marks on gill margins of a fruit body of Volvariella when it had a 10 mm diameter cap and observing the marks at maturity, when the cap diameter had increased to about 50 mm. The diagrams show the profiles of three gill plates; ink marks are shown as arcs of small black dots on the margin of the ‘immature’ gills. New tissue formed during maturation is shown shaded dark grey. The two diagrams illustrate the expected effects on the disposition of ink marks of different strategies for gill growth. If the gills grow at their margin (top panel), hyphal tips will grow beyond the ink marks and the ink will become buried (and hidden from view) within the gill spaces. If the gills extend by growth of their roots into the cap tissue (bottom panel), the original margins remain intact as the gill organisers ‘excavate’ the gill spaces. In the real experiment the ink marks remained in situ through a 5-fold increase in size, it is concluded that Volvariella gills extend at their roots; the same hyphal tips that were located at the gill margin in the immature fruit body remained at the gill margin in the mature fruit body. Diagrams modified from Moore (1995). |
It’s a simple experiment but it has decisive implications. The crucial observation is that ink marks placed on the free edges of the gills remained at the free edges even though the gills can increase in size by several mm as it matures. It means that the gill edge is fully differentiated; the gill edge is not a growth site so ink marks placed upon it are not over-grown as the gill matures because the same hyphal tips that were located at the gill margin in the immature fruit body remained at the gill margin throughout maturation. Thus, it follows from observation (b) that individual gills of Volvariella do not grow by extension at their free edge, and from (c) that the cap margin is not a growth centre for radial extension. So now you should be able to answer the question about secondary gills posed in the legend to Fig. 10.
The inevitable conclusion is that agaric gills extend in depth by growth at their roots and by insertion of hymenial elements into their central and root regions. The cap extends radially, presumably by insertion of new hyphal branches alongside the older hyphae in a broad region behind the margin. Thus, the hyphal tips which form the cap margin when it is established at the very earliest stage of development remain at the margin. They do not continue to grow apically to extend the margin radially, nor are they overtaken by other hyphae; instead they are ‘pushed’ radially outwards by the press of fresh growth behind, and they are joined by fresh branches appearing alongside as the circumference of the margin is increased. Similar experiments have been done with fruit bodies of Pleurotus pulmonarius, marked at the gill margins with the vital stain Janus Green: the originally-marked gill margins remained intact although further growth of the cap periphery involved production of entirely new (unstained) tissue which extended the cap periphery radially outwards. Evidently, these gills also grow at their root. However, in P. pulmonarius the cap margin proved to be a region of active growth, reference marks made on the margin were overtaken by growth of the tissue and the new cap margin was free of the staining added at the primordial stage (observations of Carmen Sánchez, illustrated as Fig. 6.42 in Moore, 1998a).
As we’ve noted, the young Coprinopsis fruit body has a different geometry from a conventional ‘mushroom’ because the primordial cap completely encloses the top of the stem, and primary gills, from their formation, have their inner (tramal) tissue in continuity with the outer layers of the stem. We have also stated that both stem and cap circumferences increase by a factor of around ten (more at the cap margin, slightly less at the stem). Now, if the (stem) surface to which the gills are attached is expanding, then the gills would be expected to be prone to widening during maturation (Fig. 18).
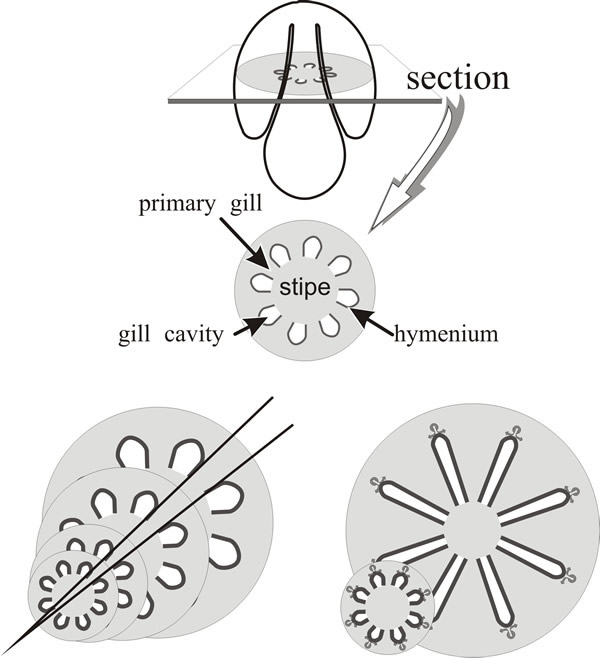 |
| Fig. 18. Primary gills of Coprinopsis are connected to the stem. The top panel is intended to orient the reader and to illustrate the basic layout of a transverse section of the primordial pileus. If these geometrical relationships remained unchanged during expansion, gill thickness would increase greatly for two reasons: (a) because the primary gills are connected to the stem circumference, increase in stem circumference would be accompanied by increase in gill thickness (bottom left panel); (b) because gill organisers migrate radially outwards (generating the branching pattern which forms the protohymenia as palisades bounding an incipient fracture plane as illustrated in Fig. 21) the gills would inevitably become thicker as their radial paths diverge (bottom right panel). The solutions to these problems are illustrated in Fig. 19. |
However, mature gills are narrow so this tendency to widen as the circumference increases must be compensated by increase in number of gills (Fig. 19). How this is accomplished is indicated in images like Fig. 20.
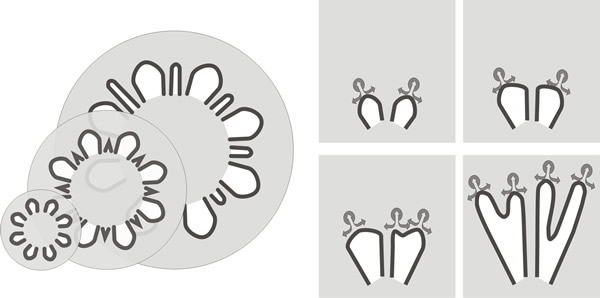 |
| Fig. 19. Generation of new primary and secondary gills in Coprinopsis. At left: increase in the thickness of the primary gill at its junction with the expanding stem is compensated by the appearance of new gill cavities within the trama of the original primary gills. At right: the formative element in outward extension of the gill is the gill organiser located in the tissue which borders the outermost part of the gill cavity. As their radial paths diverge, this part of the gill cavity expands tangentially until sufficient space exists between neighbouring gill organisers for a new organiser to appear between them. Continued outward migration of parent and daughter gill organisers creates a secondary gill between them. These two mechanisms are not alternatives; they occur together to generate the community of radial, narrow gills which characterise the mature Coprinopsis fruit body (see Fig. 20). |
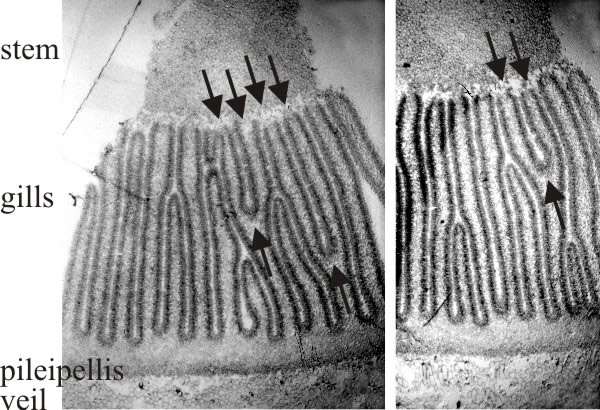 |
| Fig. 20. Two transverse sections of a fruit body cap of Coprinopsis cinerea. Stem tissue is at the top. All of the gills which have their tramal tissue continuous with the stem are primary gills. Note that two of these in one section and one in the other show a Y-shaped profile, in which both arms of the Y are still connected to the stem (arrows). This indicates that primary gills proliferate by formation of a new gill space within the trama of a pre-existing primary gill. Light micrographs by Isabelle V. Rosin. Illustration modified from Moore (1998a). |
This shows formation of a new gill cavity and its bounding pair of hymenia within the trama of a pre-existing primary gill. The Y-shaped gill structure is the crucial observation. There seems to be a formative element at the fork in the Y where the change in structure occurs from the randomly intertwined tramal hyphae with large intercellular spaces to the highly compacted hymenial plates separated by the (new) gill cavity. This has been called a gill organiser and it is presumed that a new gill organiser appears between existing hymenia as stem expansion forces them apart (and perhaps diminishes an inhibitory field to the point where a new gill organiser can form). Observation of many fruit body sections (Rosin & Moore, 1985a) shows that these Y-shaped gill structures are oriented exclusively as though the new gill organiser originates at the level of the stem surface and moves outwards towards the cap. So there is a clear indication that gills in Coprinopsis broaden radially outwards, their roots extending into the undifferentiated tissue of the cap tissue.
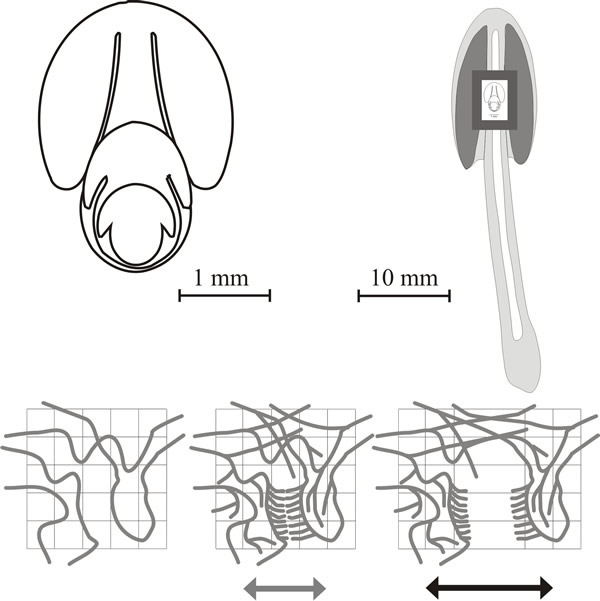 |
| Fig. 21. Dynamics of fruit body expansion in Coprinopsis cinerea. In the panel at top left outline diagrams traced from sections of fruit body initials and primordia like those shown in Fig. 12.1 are nested together to illustrate the steady outward expansion of the tissue layers. These diagrams are superimposed (to scale) onto a median diagrammatic section of a mature fruit body in the top right panel to demonstrate the extent of the outward movement of tissue boundaries. The bottom panel shows how this expansion can generate cavities by putting tension stress across an incipient fracture plane. In these diagrams, a small region of undifferentiated tissue is shown first against a reference grid. In the central diagram a cycle of branching is assumed to have taken place forming branches of determinate growth arranged in two opposing palisades (= two young hymenia). This constitutes the incipient fracture plane and when tension is applied the palisades will be pulled apart (right hand figure) producing a gill cavity bounded by two hymenia. |
In the cap tissue, it is believed that there is a gill organiser in the vicinity of each gill arch (that is, at the apex of each gill space) which is responsible for the progression of the gill morphogenetic field outward, away from the stem into the steadily replenished undifferentiated cap tissue (Fig. 21).
Formation of secondary gills is presumed to occur when the distance increases between existing gills because of cap expansion, again creating the space within which a new organiser can arise. This set of observations gives presumptive evidence for two classic components of theoretical morphogenesis (Rosin & Moore, 1985a). On the one hand we can hypothesise radial diffusion of an activating signal which assures progression of the gill sort of morphogenesis radially, on the other hand we may have tangential diffusion of an inhibitor which prevents formation of new organisers until the extent of the tissue domain exceeds the effective range of the inhibitor; at which stage a new organiser can arise in response to the radial activating signal. Interaction between the presumed gill organiser activator and the presumed gill organiser inhibitor is all that is necessary to control gill spacing, gill number, gill thickness, and the perfectly radial orientation of the gill field.
The characteristic general arrangement of the stem, gills and cap which is the essential mushroom, constitutes the basic body plan of gilled fungi. The basic mammalian body plan can provide for animals as diverse as humans, giraffes and the blue whale, yet it is arrived at by essentially identical morphogenetic processes in those animals, such that the early embryonic stages are virtually indistinguishable. In the face of this commonality it is unlikely that gilled fungi would use a multitude of morphogenetic processes to arrive at the same basic mushroom body plan, even though there are several distinct phylogenetic routes. It is most significant that the growth vector across the width of the gill is directed away from the stem in all species in which this point has been examined implying that the gill grows at its root and not at its margin. Thus, the generalised interpretation is that all gills grow by extension at the root and not by growth at the free edge (Moore, 2013).
For Coprinopsis and Volvariella, which are characteristic agarics, the developmental vector of the gills is directed away from the stem. Surprisingly, the same also seems to be true for ‘polypores with gills’ like Pleurotus and Lentinula. When gill edges of P. pulmonarius and L. edodes were marked with the vital stain Janus Green, the edge remained intact even when further growth and development was quite considerable. Evidently, these gills also grow at their root. However, outward growth of the cap margin was not the same as in agarics. In agarics the cap margin advances outwards as a result of the press of growth behind and marks made on the margin remain at the margin as the cap develops. In P. pulmonarius and L. edodes the margin proved to be a region of active growth, reference marks made on the margin were overtaken by growth of the tissue and the new cap margin was free of the staining added at the primordial stage. Presumably these different growth patterns are consequences of the suite of developmental strategies which, like cell inflation versus cell proliferation in particular, create the morphogenetic distinction between agarics and polypores.
Certainly, the radially outward growth of cap tissue evident in Pleurotus pulmonarius is functionally similar to the lateral extension of the fruit body in the resupinate polypore Phellinus contiguus (Butler & Wood, 1988). It seems, therefore, that ‘gilled polypores’ maintain the ancestral direction of development for the structural tissue of the fruit body, but abandon the ancestral direction for development of the hymenophore. So what of the pores of polypores?
Long ago Corner (1932) described how pores and dissepiments arose in an area on the underside of the young part of the cap of Polystictus xanthopus, a region he called the ‘pore field’. Pores, of course, are the tubular holes which are lined with the hymenium. The tissue which separates the pores is called the dissepiment. This word literally means a partition and is specifically assigned to the tissue between the pores of a polypore. Described in this way, it is implied that the pore is an entity in its own right. However, like the space between the gills of gilled-fungi, the pore is defined by the tissue around it; that is, by the pattern-forming growth and branching processes which take place in the hyphae of the dissepiments.
The pore is the place where the dissepiment does not grow. Corner (1932) defined the ‘pore field’ as an annulus near the margin on the underside of the cap, in which localised development of differentiating hyphal growth resulted in initiation of dissepiments as protruding ridges, which branched and united around non-extending areas. The latter became the pore bases. Corner also described a spine field in the hydnoid fruit body of Asterodon ferruginosus, corresponding to the pore field of polypores, in which spines arose as localised areas of downwards growth 200-300 µm in diameter in which the hyphal tips were positively gravitropic (growing towards the centre of the Earth).
Constituent cells of the pore wall differentiated from mycelial hyphae. Hymenial elements were the first to differentiate, basidia and setae (a seta is a stiff hair-like sterile hyphal end with thick walls, projecting from the hymenium) arose directly from 3-day-old divergently-growing mycelium, setae differentiating first. In the pore bases this sparse discontinuous hymenium became more continuous as more basidia, and also setae, differentiated in parallel with dissepiment growth (Butler, 1992a). Setae, basidia and dissepiment-building hyphae all differentiated on explants (Butler, 1992b).
Phellinus contiguus in nature produces a poroid fruit body which is flat on the substratum (that is, a resupinate fruit body), the usual substratum being the lower side of branches and twigs. Butler & Wood (1988) described production of pores on colonies cultured in Petri dishes, 3-4 mm behind the colony margin. Fruit bodies formed in agar cultures of Phellinus contiguus were generally similar to naturally-occurring specimens; even the pore size and density were within the range found in nature. Butler (1995) found that fruiting could be promoted by one or more metabolic products found in extracts of P. contiguus cultures. The fruiting inducing substance has not been characterised.
Pores were formed only in inverted cultures (agar upwards). Disorganised growth of the tissue occurred in dishes with the agar lowermost, showing that some hyphae that contribute to fruit body construction are not positively gravitropic, though extension of the dissepiments clearly is. Butler (1988) distinguished two developmental processes in pore morphogenesis which she called island initiation and aerial fascicle growth (a fascicle is a little group or bundle of hyphae).
Fruit body tissue arose as islands in the narrow initiation zone immediately behind the mycelial margin. New pores were formed by lateral extension from the island initials and the final pore density was not defined in the original pore field; rather, secondary pore formation occurred both by differential growth within thick dissepiment areas and by subdivision of primary pores. Continued growth of the dissepiment away from the supporting cap tissue delimited the pore. The edge of the dissepiment grows away from the underside of the cap, so the edge of the dissepiment grows vertically downwards. Clearly, then, pores extend at their free edge, but gills always extend at their root.
Updated July, 2019
