10.13 Secondary metabolism (including commercial products like statins and strobilurins)
Primary and secondary metabolisms are coextensive; they can occur at the same time in the same cell and draw carbon-containing intermediates from the same sources. Generally, though, as nutrients are depleted the rate of growth slows and eventually stops. The progress of metabolism is correspondingly altered and a number of special biochemical mechanisms appear (or become amplified) to establish what is called secondary metabolism. The resultant secondary metabolites seem to be unnecessary for normal growth of the organism, but rather appear to be aimed at functions such as intercellular communication and defence and competition with other organisms.
Secondary metabolism is a common feature of fungi. It consists of a relatively small number of enzymological processes (often of relatively low substrate specificity) which convert a few important intermediates of primary metabolism into a wide range of products (Bu’Lock, 1967). These later stages of secondary metabolism are so varied that individual secondary metabolites can have a narrow species distribution. The subject is huge, and this section is not intended to rival the comprehensive treatment which you can find in texts like Mérillon & Ramawat (2017) and Bills & Gloer (2016), rather we intend to illustrate the chemical versatility of fungi with a few examples that have some sort of importance to our daily lives.
The majority of secondary metabolites have their origins in a small number of the intermediates in pathways dealt with above:
- acetyl CoA is, perhaps, the most important, being a precursor for terpenes, steroids, fatty acids and polyketides;
- phosphoenolpyruvate and erythrose 4-phosphate initiate synthesis of aromatic secondary metabolites through the shikimic acid pathway by which aromatic amino acids are synthesised;
- further secondary metabolites are derived from other (non-aromatic) amino acids (Fig. 14).
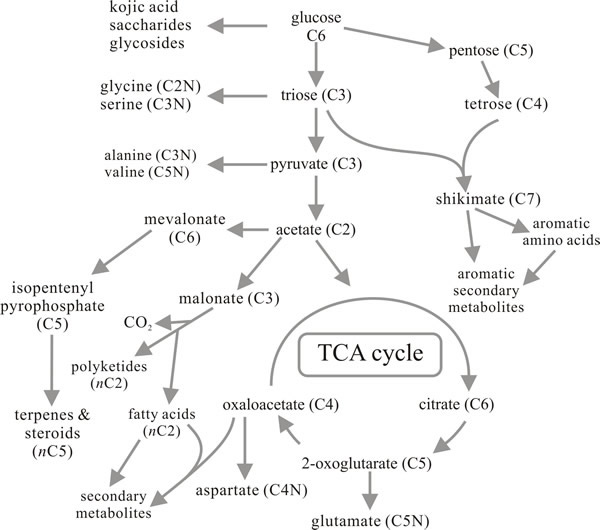 |
Fig. 14. Metabolic route map summarising the
relationships between primary metabolism and the major pathways for
synthesis of secondary metabolites. Compare with Figs 9 and 12. Modified
from Moore, 1998. |
Terpenes, carotenoids and steroids are widely distributed in nature and though the fungal products are not unusual in that sense, many of the end-products have chemical structures which are unique to fungal metabolism. All of these compounds are related by having five carbon atoms arranged as in the hydrocarbon isoprene. The terpenes are the simplest of the naturally occurring isoprenoid compounds and are derived by condensation of a precursor isoprene unit to form an isoprenoid chain (Fig. 15).
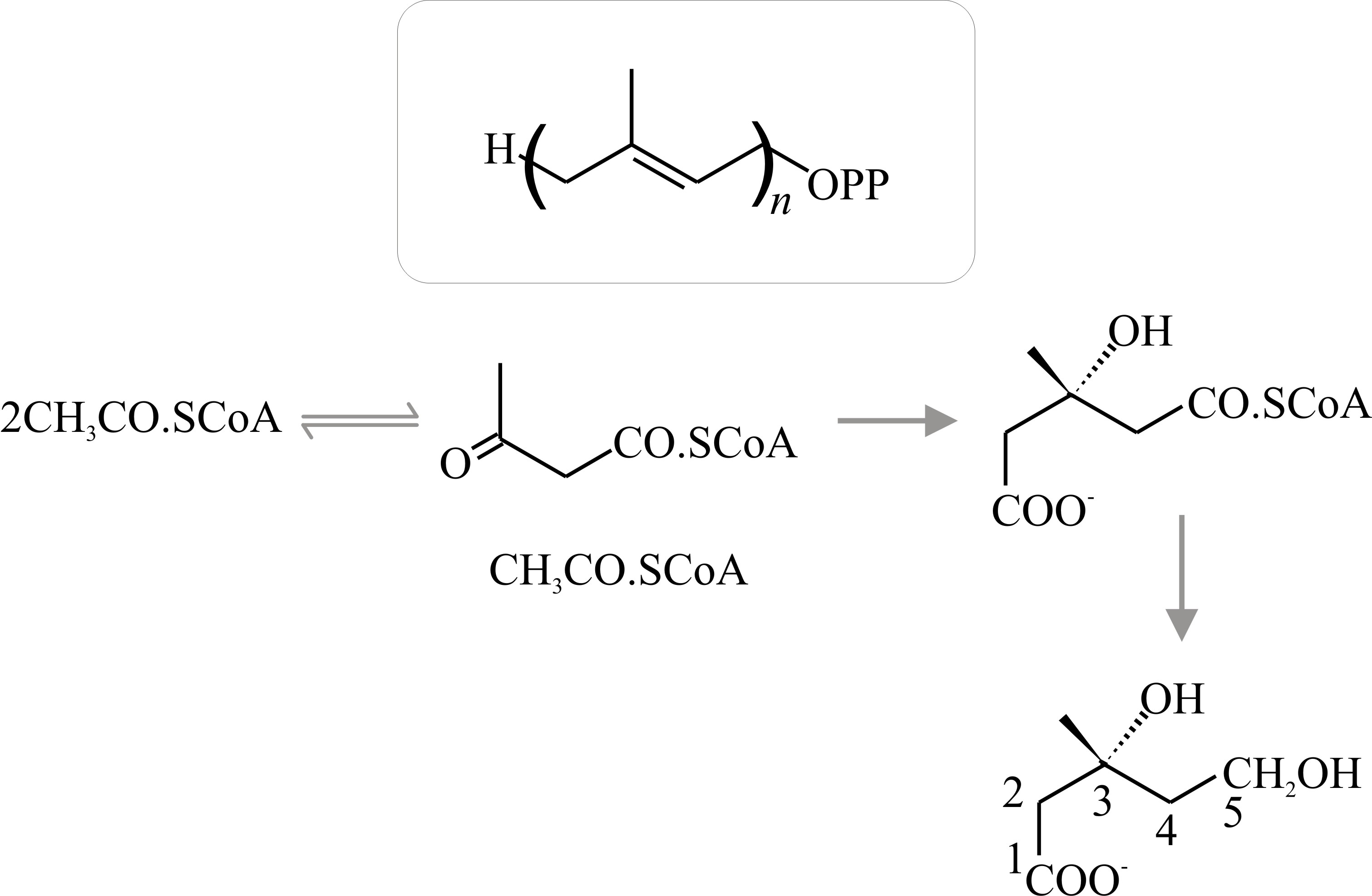
Strictly, terpene is the name of the hydrocarbon with the formula C9H16, but the term is used generally to refer to the ten-carbon isoprenoids which may be open-chain (that is, acyclic) or cyclic compounds. Synthesis of acyclic terpenes starts when dimethylallylpyrophosphate condenses with a molecule of isopentenyl-pyrophosphate to form the 9-carbon monoterpene geranylpyrophosphate (Fig. 16); addition of another isopentenylpyrophosphate gives the 15-carbon sesqui- (one-and-a-half) terpene farnesylpyrophosphate; and of another, the 20-carbon diterpene geranylgeranylpyrophosphate, and so on.
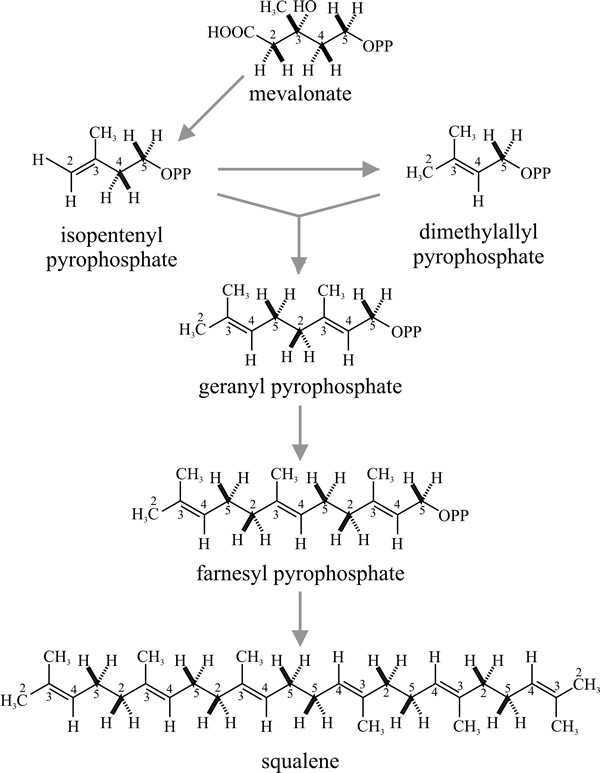 |
Fig. 16. Synthesis of acyclic (that is, straight
chain) terpenes by successive condensations. Dimethylallyl-pyrophosphate
condenses with isopentenyl-pyrophosphate to form geranylpyrophosphate.
Squalene is the precursor of sterols (see Fig. 19). Squalene is formed
by head-to-tail condensation of two sesquiterpene
(farnesylpyrophosphate) units. Check the numbering on the carbon atoms
to understand how these molecules are assembled. Modified from Moore,
1998. |
As many as 24 isoprene units may condense in this way, though secondary metabolites usually have between two and five. Not all such molecules are secondary metabolites; the ubiquinones of the respiratory chain are polyprenoid quinones. Sesquiterpenes are the largest group of terpenes isolated from fungi. Most of the fungal sesquiterpenes are based on carbon skeletons which can be derived by cyclisation of farnesylpyrophosphate as shown in the schemes in Fig.17 (most of which are plausible possibilities rather than defined metabolic pathways).
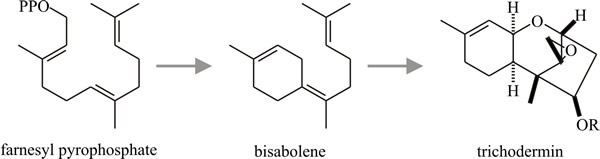 |
Fig. 17. Sesquiterpenes:
possible cyclisations of farnesylpyrophosphate to produce the
trichothecane nucleus. Modified from Moore, 1998. |
Diterpenes are derived by cyclisation of geranylgeranyl pyrophosphate (Fig.18) and include the gibberellins, a mixture of plant growth promoters which were first isolated from culture filtrates of the plant pathogen Gibberella fujikuroi (the sexual form, or teleomorph, of Fusarium moniliforme) and only later shown to be endogenous plant hormones.
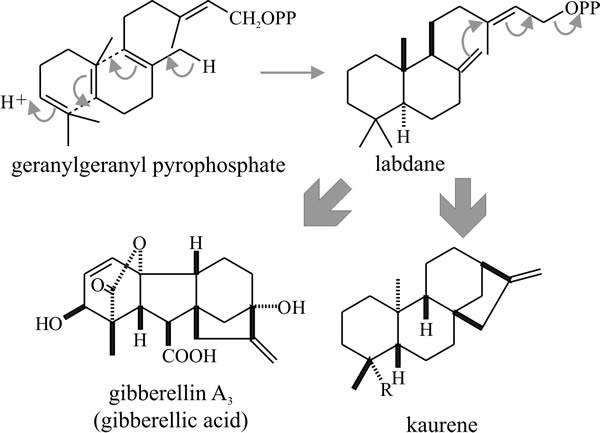 |
Fig. 18. Cyclisation of the diterpene geranyl-geranyl
pyrophosphate producing gibbane and kaurane structures. Gibberellic acid
is a plant growth hormone that was discovered only after it had been
isolated as a secondary metabolite of a pathogenic fungus that caused
growth abnormalities in its plant host. Modified from Moore, 1998. |
The major significance of the triterpenes (C30) is that the acyclic triterpene squalene is the precursor of sterols. Squalene is formed by a head-to-tail condensation of two sesquiterpene (farnesylpyrophosphate) units; it is so called because it is found in high concentration in shark liver oil (Squalus is a genus of dogfish sharks), but in fungi as well as in other organisms sterols (and cyclic triterpenes) are derived by cyclisation of squalene oxide (Fig. 19).
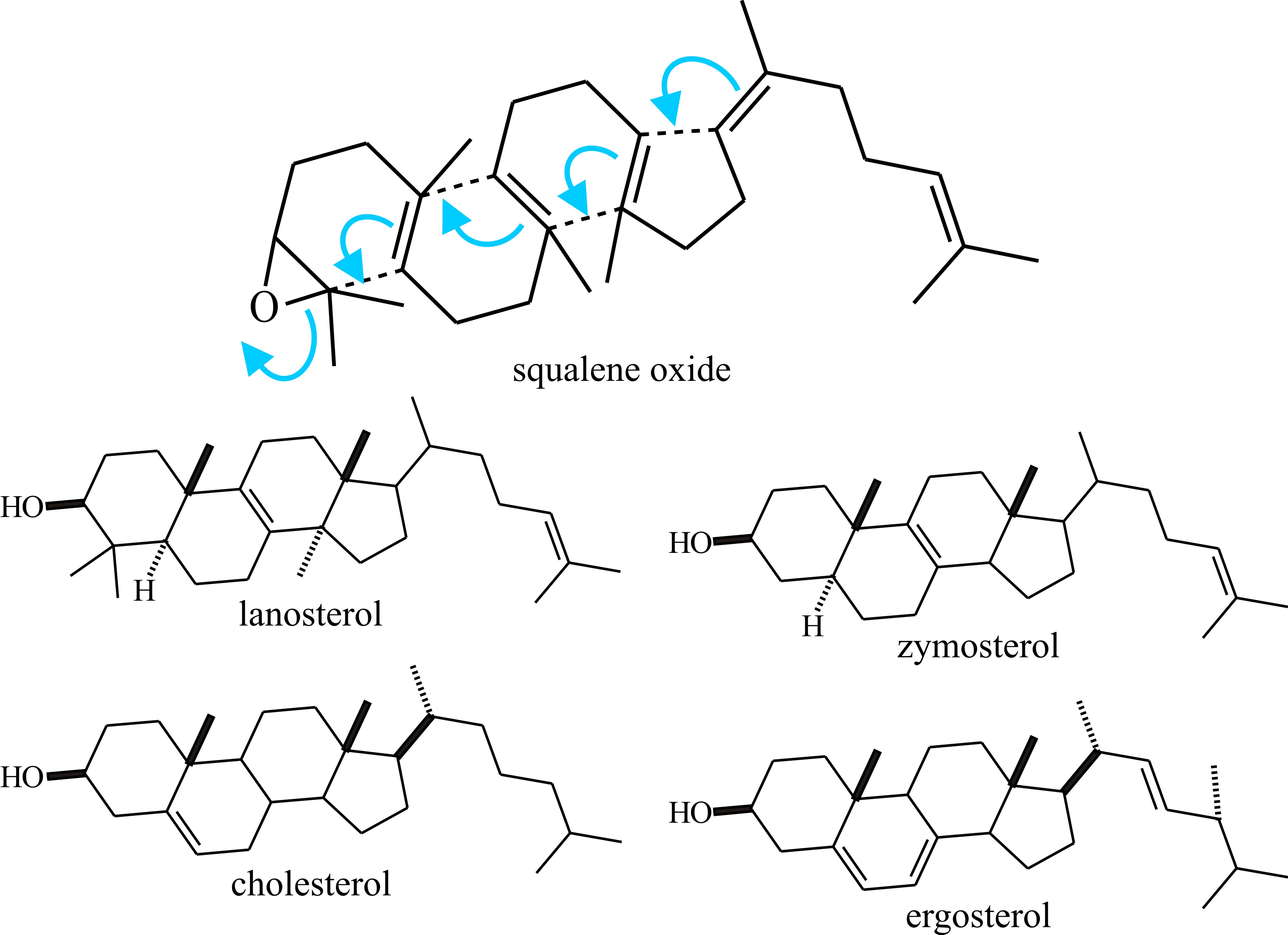 |
Fig. 19. Sterols (and cyclic triterpenes) are derived
by cyclisation of squalene oxide. This is illustrated in the upper panel
and structural formulae of some sterols are shown below. More details
about the clinical importance of differences between cholesterol and
ergosterol can be found in the first section of Chapter 18. Modified
from Moore, 1998. |
Cholesterol is the quantitatively predominant sterol in animals where it serves to control membrane fluidity. Ergosterol, so named because it was first isolated from the ergot fungus (Claviceps purpurea), probably fulfils a similar role in fungi, influencing permeability characteristics of the membrane and as it is unique to fungi; ergosterol synthesis is a potential target for antifungal agents. However, other sterols, even cholesterol, occur commonly and a very wide range of sterols has been detected in fungi which have scope for characterising strains. Lipid, sterol and phospholipid contents differ between yeast and mycelial forms of Candida albicans and Mucor lusitanicus, so there is a morphogenetic connection, too. The sequential order of steps in sterol synthesis is well established. A crucial step in sterol synthesis is controlled by the enzyme b-hydroxy-b-methylglutaryl-CoA reductase (HMG Co-A reductase), which converts HMG-CoA to mevalonic acid.
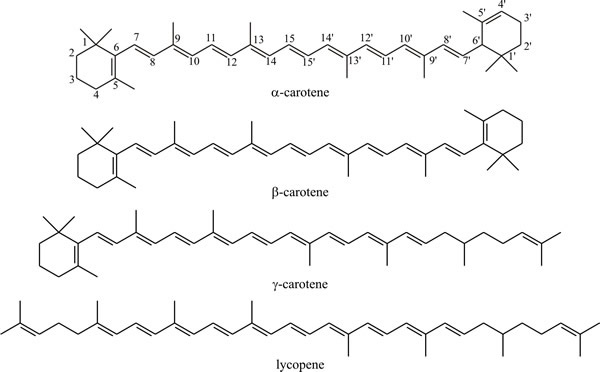
In much the same manner as squalene is formed by head-to-tail condensation of sesquiterpenes, the C40 carotenoid pigments are formed by head-to-tail condensation of two diterpenes. These include acyclic compounds like lycopene, the pigment responsible for the red colour of tomatoes and monocyclic and dicyclic compounds like α-, β- and γ-carotene (Fig. 20). The carotenes and some keto-derivatives are widely synthesised and carotenoids are good taxonomic markers for some fungi, though not all fungi produce them; the biosynthetic pathway is well established.
 |
Continued condensation of isoprene units can produce terpenes with very long carbon chains. Natural rubber is a highly polymerised isoprene compound, containing of the order of 5000 isoprene units. The latex of the major commercial source, the rubber palm Hevea brasiliensis, is an aqueous colloidal suspension of particles of this hydrocarbon. In strong contrast, the milk-like juice exuded from broken fruit bodies of Lactarius, stems of Mycena and gill edges of Lacrymaria is sometimes also called ‘latex’ but is chemically very different from rubber palm latex, being only superficially similar in appearance. The fungal product probably differs in structure between genera, but the ‘latex’ or ‘milk’ of Lactarius rufus has been shown to contain mannitol, glucose and lactarinic acid [CH3(CH2)11CO(CH2)4COOH; structural formula shown in Fig. 25]. The latter is a modified fatty acid (6-oxo-octadecanoic acid, also known as 6-ketostearic acid).
More secondary metabolites are synthesised through the polyketide pathway in fungi than by any other pathway. Polyketides are characteristically found as secondary metabolites among Ascomycota; they are rarely encountered in Basidiomycota and are produced by only a few organisms other than fungi. Polyketides are more correctly described as poly-β-ketomethylenes, the fundamental acyclic chain from which they are derived being comprised of -CH2CO- units. Just as linear poly-isoprenoid chains can ‘fold’ and cyclise, so too the polyketides can cyclise to produce a wide range of different molecules (Fig. 21). As might be expected from the chemical nature of the repeating unit, synthesis of polyketides involves transfer of acetyl groups which are ultimately derived from acetyl-CoA. In fact the synthesis of polyketides has a lot in common with fatty acid biosynthesis.
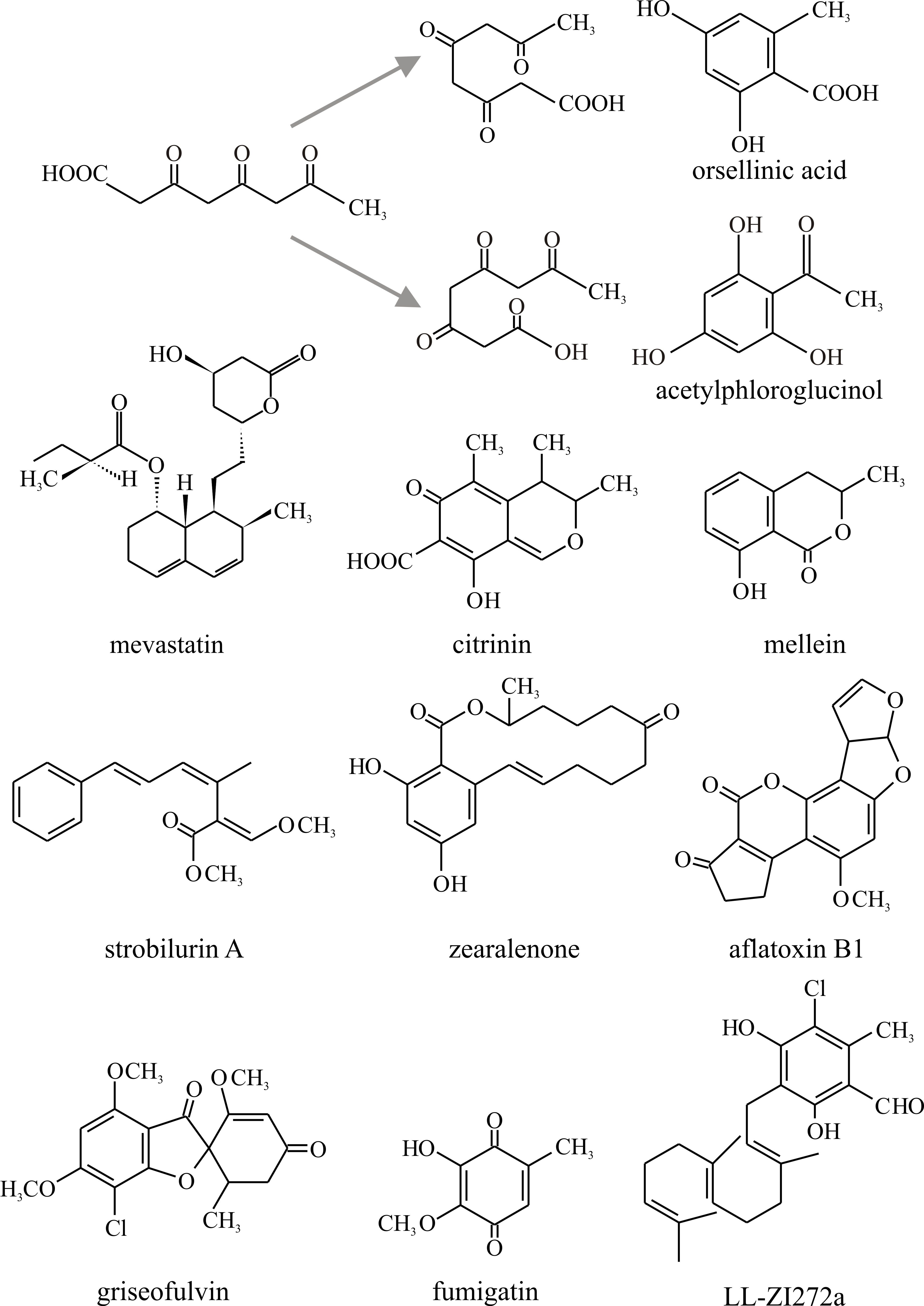 |
Fig. 21. Polyketide chains can fold in a variety of
ways and internal aldol condensations form closed aromatic rings. To
illustrate this, the alternative cyclisations of a tetraketide are shown
at the top, together with the potential products orsellinic acid and
acetylphloroglucinol. The bottom section shows the structures of a small
selection of polyketides discussed in the text. Modified from Moore,
1998. |
Fatty acids are catabolised by stepwise removal of ‘acetyl-units’ (β-oxidation, see Fig. 11) but their synthesis requires malonyl-CoA which is formed by carboxylation of acetyl-CoA by the enzyme acetyl-CoA carboxylase:
CH3COSCoA + HCO3- + ATP → COOHCH2COSCoA + ADP + Pi
Synthesis of fatty acids is carried out by a complex of enzymes called the fatty acid synthetase system. In the first reaction ‘acetate’ from acetyl-CoA is condensed with a malonyl group (from malonyl-CoA) to form acetoacetate which is still bound to the enzyme and then reduced, dehydrated and reduced again to form a butyryl-enzyme complex [CH3CH2CH2CO-S-enzyme]. For chain lengthening, the butyryl residue is condensed with another malonyl group followed by repetition of the reduction, dehydration, reduction cycle. Thus, malonyl-CoA provides all the carbon of long chain fatty acids except for the two terminal atoms, which derive from the ‘acetate’ initially introduced from acetyl-CoA.
This brief description of fatty acid synthesis can be echoed by a description of polyketide synthesis which is initiated by condensation of an acetyl unit with malonyl units, requires the respective CoA derivatives, and seems to occur on the enzymes involved. In both pathways, free precursors are not found in the cytoplasm. The chain-building mechanism is similar between fatty acids and polyketides, but for polyketides the reduction process does not occur and successive rounds of condensation generate polymers with the -CH2CO- (‘ketide’) repeating unit. The number of these can be used to designate the chain formed as, for example:
- tetraketides (examples are orsellinic acid, found in many lichens, and fumigatin, characteristically produced by Aspergillus fumigatus);
- pentaketides (for example citrinin, an antifungal agent that inhibits HMG Co-A reductase, originally isolated from P. citrinum, but commonly found in Ascomycota; and mellein, which may have value as a bioherbicide);
- heptaketides (such as the antifungal agent griseofulvin, originally isolated from Penicillium griseofulvum, which finds commercial use in treating fungal diseases of animals and plants).
The methylene (-CH2-) and carbonyl (=C:O) groups of these chains can interact in internal aldol condensations to form closed aromatic rings (Fig. 21). A great variety of cyclisations thus become possible and this, in part, accounts for the wide range of polyketides which are encountered; the number is further increased by subsequent chemical modifications. The result is that polyketide secondary metabolites are far too numerous to document here (see Agarwal & Moore, 2014) but they include many toxins, some of which have medical importance (Fig. 21).
Notable examples in Fig. 21 are:
- Zearalenone, a toxin produced in stored grain contaminated by Fusarium, and the aflatoxins, which are produced in mouldy groundnut meal by Aspergillus species and are among the most toxic, mutagenic, and carcinogenic natural products known.
- Compounds now known as statins (such as Mevastatin, Fig. 10.21), from Penicillium citrinum, and a similar molecule, later named Lovastatin, from Aspergillus terreus, originally isolated for their ability to inhibit lipid synthesis. These are HMG-CoA reductase inhibitors, and as this is the rate-limiting enzyme of the mevalonate pathway of cholesterol synthesis they are used to lower cholesterol levels in people with, or at risk of, cardiovascular disease. Inhibition of this enzyme in the liver stimulates low-density lipoprotein (LDL) receptors, resulting in an increased clearance of LDL from the bloodstream and a decrease in blood cholesterol levels. The first positive results of treatment can be seen after just one week of use. These are the wonder drugs of the 21st century (antibiotics being the wonder drugs of the 20th century) and are currently the most widely used pharmaceutical agents in the world.
- Strobilurin A (Fig. 10.21) represents the strobilurin fungicides. It is the original, natural strobilurin, produced by the pine cone fungus, Strobilurus tenacellus, a mushroom fungus that colonises pine cones and produces strobilurins to keep the pine cone to itself. Natural strobilurins are unstable in the presence of light, but there are many synthetic analogues that are more stable. Synthetic strobilurins are now the most widely used agricultural fungicides (Balba, 2007).
- Other, more complex compounds also arise and there are some compounds in which polyketide-derived aromatic rings are attached to sesquiterpenes. Examples are a group of compounds called ascochlorins that inhibit mitochondrial electron transport (Berry et al., 2010).
The primary role of the shikimate-chorismate pathway is the synthesis of the aromatic amino acids phenylalanine, tyrosine and tryptophan (Fig. 14), but the pathway provides intermediates for the synthesis of other aromatic compounds as secondary metabolites. Plants synthesise a particularly large variety of compounds by this route, though it is not so widely used in fungi, where the polyketide pathway is used more to make aromatic ring compounds. Nevertheless, numerous shikimate-chorismate derivatives that commonly occur in plants have been isolated from fungi, such as gallic acid, pyrogallol, and methyl p-methoxycinnamate (Fig. 22).
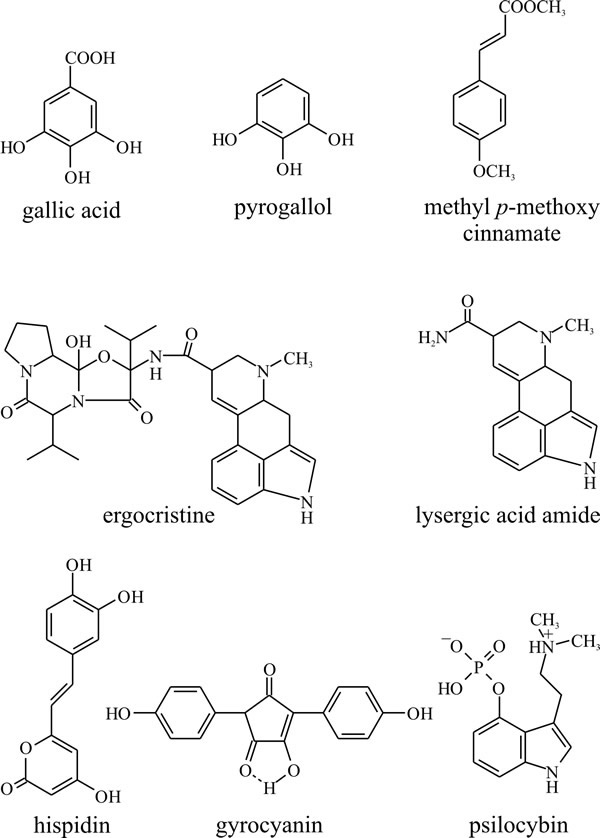 |
Fig. 22. The shikimate-chorismate pathway produces a
variety of aromatic compounds as well as the amino acids phenylalanine,
tyrosine and tryptophan. Gallic acid, pyrogallol, and methyl p-methoxycinnamate
are relatively simple compounds produced by many plants as well as
fungi. Ergocristine, which is one of the ergot alkaloids (from
Claviceps purpurea), lysergic acid amide and psilocybin, the
halucinogenic principle of the original ‘magic’ mushroom (Psilocybe),
are all essentially tryptophan derivatives. Gyrocyanin is a product of
Gyroporus cyanescens which oxidises to a blue pigment in injured
fruit bodies, and hispidin is the precursor of a polymer which seems to
be responsible for toughening the fruit bodies of Polyporus hispidus.
Modified from Moore, 1998. |
Among compounds more particularly associated with fungi are the ergot alkaloids (from Claviceps purpurea) which include ergocristine and lysergic acid amide and psilocybin, which is the hallucinogenic principle of the original ‘magic’ mushroom (Psilocybe). All of these are essentially tryptophan derivatives. Also, basidiomycete pigments like gyrocyanin, which oxidises to a blue pigment in injured fruit bodies of Gyroporus cyanescens, and hispidin, a polymer of which may be responsible for toughening of the fruit bodies of Polyporus hispidus (Bu’Lock, 1967) are derivatives of the shikimate-chorismate pathway.
Non-aromatic amino acids may also be modified: the muscarines and muscaridines (Fig. 23), which are the main toxic constituents of Amanita muscaria (but are also found in Inocybe and Clitocybe species), are synthesised from glutamate. Agaritine and related molecules like glutaminyl hydroxybenzene (or GHB) from Agaricus bisporus, the cultivated mushroom, are substituted (strictly, N-acylated) glutamate molecules, while the antifungal agent variotin (from Paecilomyces varioti) is γ-aminobutyric acid (GABA, shown in Fig. 9) N-acylated with a hexaketide (Fig. 23). Volatile amines formed by decarboxylation of neutral amino acids form part of the distinctive odours of some fungi; the fungus causing stinking smut of wheat (Tilletia tritici) produces large quantities of trimethylamine, and the aroma of Camembert cheese depends on the formation of N-dimethyl methioninol by Penicillium camemberti.
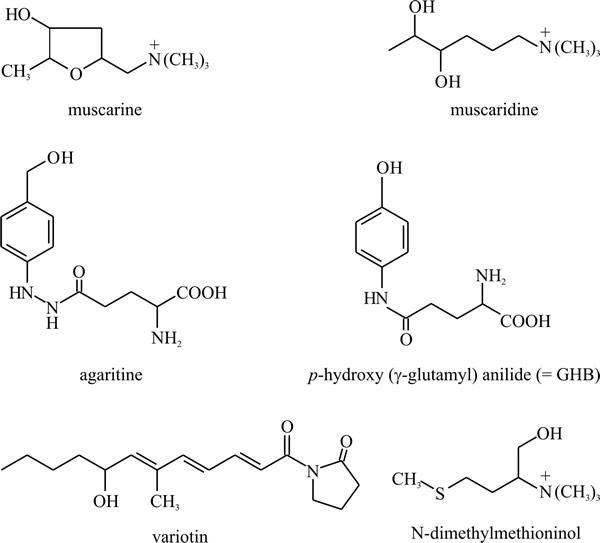 |
Fig. 23. Non-aromatic amino acids may also be modified
and accumulated as secondary metabolites. Muscarine and muscaridine, the
main toxins of Amanita muscaria, are synthesised from
glutamate, and agaritine and p-hydroxy (γ-glutamyl) anilide
(also known as glutaminyl hydroxybenzene, or GHB) from Agaricus
spp., are N-acylated glutamate molecules. Agaritine may account for up
to 0.3% of the dry weight in A. bisporus fruit bodies and GHB
may be involved in controlling basidiospore dormancy and is the most
likely precursor for Agaricus melanin in the spore walls.
Variotin is an antifungal agent isolated from Paecilomyces
varioti and is γ-aminobutyric acid N-acylated with a hexaketide.
N-dimethylmethioninol is a volatile amine formed by decarboxylation of
methionine by Penicillium camemberti. It is responsible for the
aroma of Camembert cheese. Modified from Moore, 1998. |
A variety of fungal secondary metabolites are derived from peptides; two or more amino acids linked through a peptide bond. Among those derived from dipeptides are the penicillins and cephalosporins. These could rate as the most important compounds ever to be isolated from fungi as they gave rise to a new era in medicine and a new branch of biotechnology. The basic structure of both antibiotics is derived from cysteine and valine (Fig. 24) though the variable acyl group may come from another amino acid as in penicillin G where the acyl group is phenylalanine (illustrated in Fig. 24). Biosynthesis involves the tripeptide δ-(L-α-aminoadipyl)-L-cysteinyl-D-valine, which is also illustrated in Fig. 24, but does not involve ribosomes. Instead, amino-acid activating domains of peptide synthetases determine the number and order of the amino acid constituents of the peptide secondary metabolites. In vitro reconstruction of the gene sequences of these multifunctional enzymes produces hybrid genes that encode peptide synthetases with altered amino acid specificities able to synthesise peptides with modified amino acid sequences.
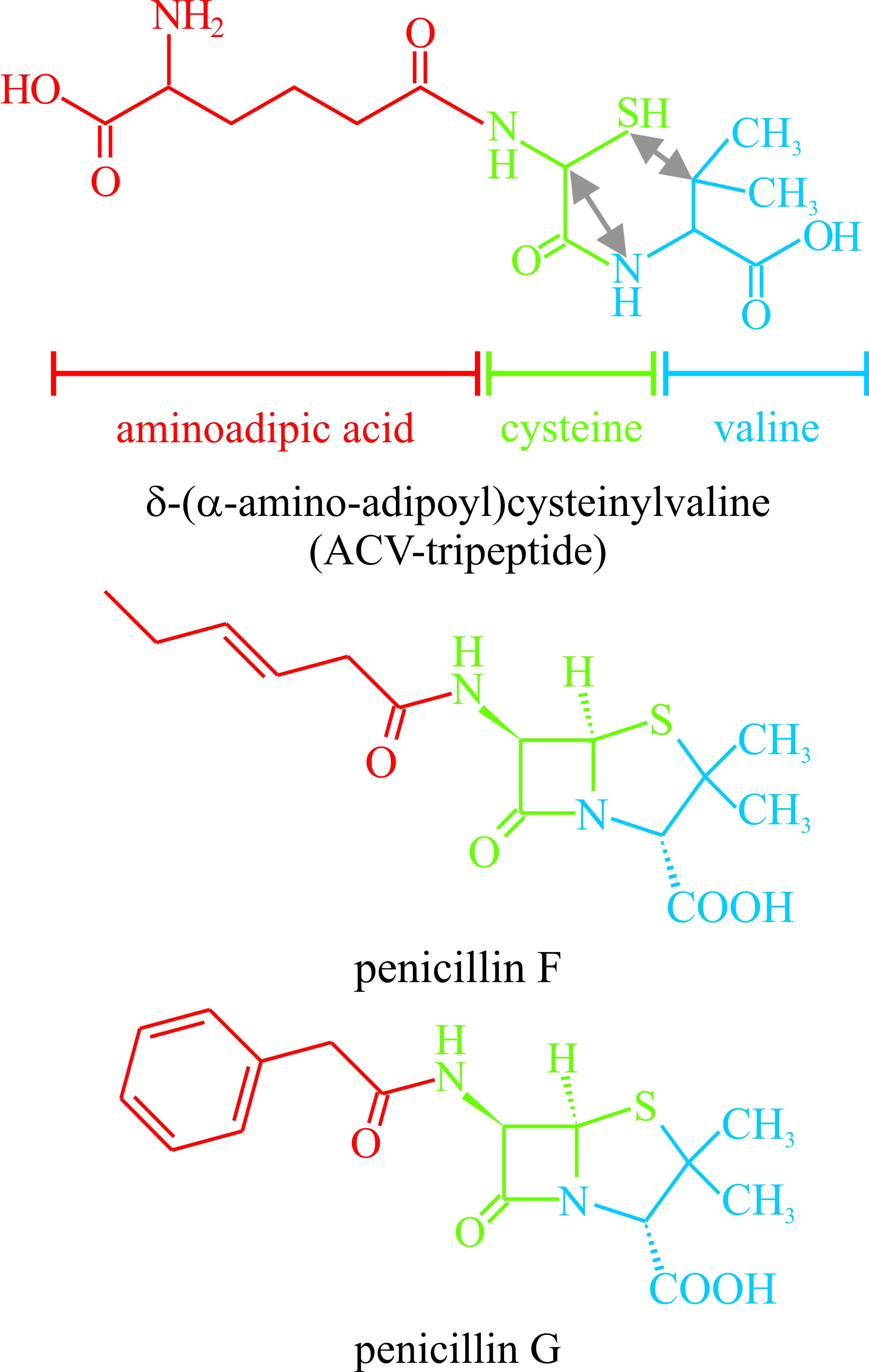 |
Fig. 24. The penicillin antibiotics are representative
of secondary metabolites which are derivatives of peptides. This figure
shows the structural formulae of two penicillins. At the top is the
precursor δ-(α-amino-adipoyl)cysteinylvaline. The aminoadipoyl, cysteine
and valine residues of this compound are identified by colour coding and the
double-headed arrows show the bonds which have to be made to create the
penicillin nucleus. See also Figs 20 and 21 in Chapter 17 for more
details. |
Also derived from peptides are the Amanita toxins of which there are many, but which can be represented by α-amanitin and phalloidin. Because of the resemblance between Amanita phalloides and edible field mushrooms, these toxins are involved in the majority of cases of mushroom poisoning. Many fungi produce siderophores for acquiring iron which, though an essential nutrient, is not readily available in aquatic or terrestrial environments or in animal hosts. These iron-binding compounds also originate as peptides formed from modified amino acids.
Chemical modification of fatty acids produces a variety of secondary metabolites, most particularly the polyacetylenes, many of which have been obtained from basidiomycetes. These compounds have straight carbon chains varying between C6 and C18, though C9 and C10 are most common in fungi. They are conjugated acetylenes (i.e. with triple bonds between adjacent carbons, e.g. hexatriyene from Fomes annosus, Fig. 25), or systems containing both ethylenic (i.e. with double bonds between carbon atoms) and acetylenic structures (e.g. nemotinic acid, Fig. 25). Polyacetylenes are derived from fatty acids by a series of dehydrogenation reactions (Negri, 2015). Also formed from fatty acids are cyclopentanes like brefeldin, which has been extracted from Penicillium, Nectria and Curvularia species (Fig. 25). Reference was made to Lactarinic acid above but if you want more specifics view: http://www.chemspider.com/Chemical-Structure.321288.html; and if you ever need authoritative chemical information go to the Royal Society of Chemistry website at http://www.chemspider.com/Default.aspx.
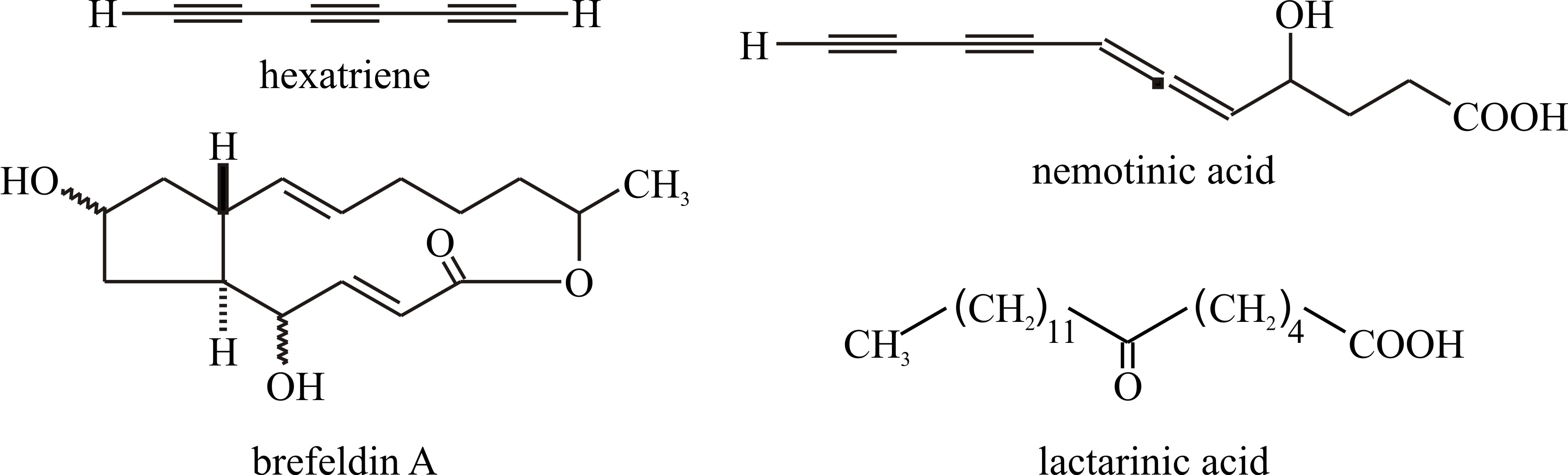 |
Fig. 25. Chemical modification of fatty acids produces
a variety of secondary metabolites including polyacetylenes and
cyclopentanes.
Lactarinic acid is a major component of the
‘milk’ or ‘latex’ which exudes from injured fruit bodies of
Lactarius spp. Lactarinic acid is 6-ketostearic acid and is very
different from true latex from the rubber tree which is an isoprene
compound. Modified from Moore, 1998. |
As must be clear from the above discussion, fungi are prolific producers of secondary metabolites and these show a variety of biological activities. Advances in genome sequencing have shown that fungal genomes harbour far more gene clusters able to synthesise secondary metabolites than are expressed under normal laboratory conditions. Filamentous fungi have already played such an important role in the history of drug discovery and development; examples being antibiotics such as penicillin, immunosuppressants such as cyclosporine, antifungals such as griseofulvin and the echinocandins, and antihypercholesterolemic drugs such as lovastatin. Because of these past (mostly accidental) successes activation of these ‘silent’ gene clusters is an on-going challenge in the hope of discovering new natural products in the future (Brakhage & Schroeckh, 2011; Brakhage, 2013; Yaegashi et al., 2014; Macheleidt et al., 2016; Rodrigues, 2016).