Differentiation of hyphae gives rise to an almost infinite variety of hyphal and cell shapes that have:
-
attracted the attention of taxonomists over the years, who have used this cellular diversity to define taxa of various ranks, and
-
been given an equally wide variety of descriptive names.
The prime source for explanation of names and terms in mycology is the
Dictionary of the Fungi (Kirk
et al., 2008):

Kirk, P.M., Cannon, P.F., Minter, D.W. &
Stalpers, J.A. (2008). Dictionary of the Fungi,
10th edn. Wallingford: CABI Publishing. ISBN-10: 0851998267, ISBN-13:
978-0851998268. Kindle edition:
ASIN: B00K7ANXJI.
A good alternative is the Illustrated Dictionary of Mycology (Ulloa & Hanlin, 2012):
Ulloa, M. & Hanlin, R.T. (2012).
Illustrated Dictionary of Mycology, Second Edition.
APS Press. ISBN: 978-0-89054-502-7. DOI:
https://doi.org/10.1094/9780890545027.
In this Resources Box we use extracts from the
Dictionary of the Fungi to show you both something of the range of diversity that has been observed in fungal cells and the usefulness of this reference source. We have corrected typographical errors in the text, but not otherwise changed the Dictionary text. In particular, we maintain the Dictionary's numbering of the figures (though we have broken up some of the larger figures to make them more display-friendly).
You can find explanations for technical terms not explained in the text
by referring to the Dictionary of the Fungi, 10th Edition,
where you will also find explanations of the abbreviations
used in the reference citations in this text,
although we have added some live hyperlinks to DOIs and other
URLs for your convenience.
We have arranged the extracts to tell a particular story
(in the Dictionary they are in alphabetical order). Our arrangement
attempts to show that the hyphal extension growth we have described in earlier sections is modified, directed and controlled to produce an enormous range of fungal cell types
that serve particular functions, and the resulting morphological
diversity can be (and has been) used to classify fungi systematically.
You can read the page as it is laid out to receive our overall message,
or you can navigate to specific entries on our page with the internal hyperlinks
in the following contents list (you'll find that each section ends with a 'return to top' hyperlink that returns you to this point):
Conidial nomenclature
conidiogenous
conidiophore
wall-building
Fig. 41. Wall building in relation to conidiogenesis
Fig. 1. Conidiogenous events
Fig. 2. Conidiogenous events
Fig. 3. Conidiogenous events
conidioma
Fig. 13. Conidiomatal types
ascus
bitunitate asci
Fig. 4. I. Predehiscence stage of asci
Fig. 4. II. Dehiscence stage of asci
Fig. 5. Ascus apex components
tissue types
Fig. 38. Hyphal tissue (textura) types
dolipore septum
Fig. 16. Summary diagram of the several forms of the dolipore/parenthesome septum in Basidiomycota
basidium
Fig. 9. Basidiospore development (diagrammatic)
Fig. 10. Basidium terminology
Fig. 11. Basidial types
cystidium
Fig. 15. Cystidia
Hyphal analysis
Fig. 19. Hyphal types
Fig. 20. Hyphal systems
hyphidium
Fig. 21. Hyphidia
Conidia and conidiogenesis
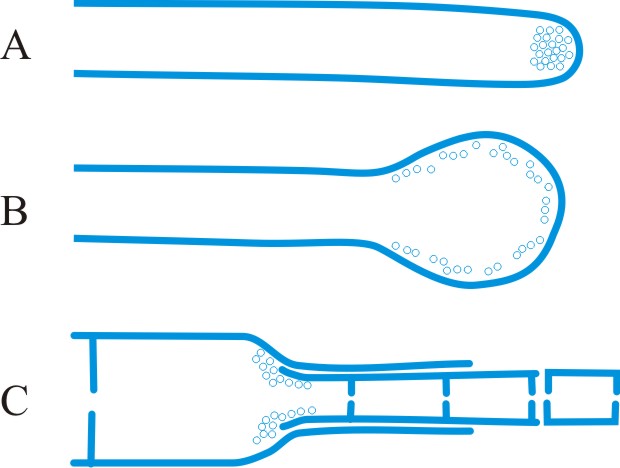
Conidial nomenclature. The traditional approach to the nomenclature of the spores of anamorphic fungi is that of Saccardo who differentiated seven morphological types (amero-, dictyo-, didymo-, helico-, phragmo-, scoleco- and staurospores), based on shape and septation, the terminology for which was further qualified according to whether the spores were pigmented (phaeoamerospores, etc.) or not (hyaloamerospores, etc.); see Anamorphic fungi. Kendrick & Nag Raj (in Kendrick
(ed.), The whole fungus 1: 43, 1979 [https://doi.org/10.1002/fedr.19800910312]) discussed Saccardo’s categories and offered precise definitions. Since Hughes (CJB 31: 577, 1953) drew attention to the systematic importance of conidiogenous events (q.v.), a nomenclature for conidia has evolved based on the methods by which the conidia develop, giving rise to terms such as annelloconidia, phialoconidia, tretoconidia etc. (cf.). These are not universally accepted, the preferred usage now being ‘conidium’ with qualifying adjectives or descriptors. Main contributions to conidial terminology have been made by the first Kananaskis Workshop-Conference in 1969 (see Kendrick (ed.), Taxonomy of fungi imperfecti, 1971
[https://trove.nla.gov.au/version/25412018] ); Ellis (Dematiaceous hyphomycetes, 1971
[URL:
https://www.cabdirect.org/cabdirect/abstract/19721100118]) offered a series of definitions as did Kendrick & Carmichael (in Ainsworth et al. (eds), The Fungi 4A: 323, 1973
[ISBN-10: 0120456044, ISBN-13: 9780120456048]), Cole (CJB 53: 2983, 1975
[https://doi.org/10.1139/b75-328]) and Cole & Samson (Patterns of development in conidial fungi, 1979
[ISBN-10: 0273084070; ISBN-13: 978-0273084075]).
The descriptive terminology in both conidial morphology and conidiogenous events was initiated by Minter et al.
in the following three references:
developed by Sutton (in Reynolds & Taylor (eds), The fungal holomorph: 28, 1993
[ISBN-10: 0851988652, ISBN-13: 978-0851988658]) and Hennebert & Sutton, and Sutton & Hennebert (in Hawksworth (ed.), Ascomycete systematics: 65, 77, 1994
[ISBN-13: 9781475792928]), and is used in this edition of the Dictionary. Return to top.
conidiogenous, producing conidia; - cell, any cell from or within which a conidium is directly produced; - locus, the place on a conidiogenous cell at which a conidium arises; Kendrick (1971: 258
[https://trove.nla.gov.au/version/25412018). Return to top.
conidiophore, a simple or branched hypha (a fertile hypha) bearing or consisting of conidiogenous cells from which conidia are produced; sometimes used when describing reduced structures for the conidiogenous cell. Return to top.
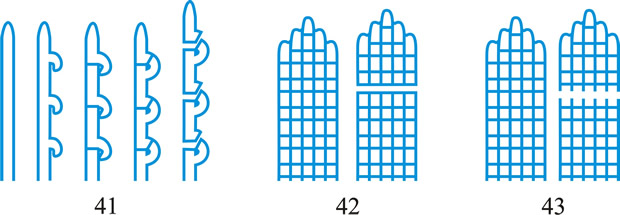
wall-building, descriptive of hyphal growth in which cell wall material is produced by certain ultrastructural secretory bodies in the cytoplasm. Three types of wall-building may be distinguished (Fig. 41): apical, in which the bodies are concentrated at the hyphal tip, producing new wall by distal growth, forming a cylindrical hypha in which the youngest wall material is at the tip; ring, in which the bodies are concentrated adjacent to the cell wall at some point below the tip, in the shape of an imaginary ring, producing new wall by proximal growth, forming a cylindrical hypha in which the youngest wall material is always at the base; diffuse, in which the bodies occur throughout the cytoplasm at a low concentration, producing lateral growth (i.e. swelling of the cylindrical hypha) by alteration of pre-existing wall. The terms assume special significance in conidial development where they have been used to clarify the concepts of thallic and blastic. Apical wall-building occurs in Geniculosporium, Cladosporium and Scopulariopsis, and ‘phialides’ where conidia are produced in gummy masses (e.g. Trichoderma) or false chains (e.g. Mariannaea). Ring wall-building occurs in ‘phialides’ with conidia in true chains (e.g. Penicillium, Chalara), in so-called meristem arthrospores (e.g. Wallemia) and in conidiogenous cells of basauxic fungi (e.g. Arthrinium). Diffuse wall-building occurs simultaneously with or shortly after apical or ring wall-building in most of the preceding examples, but its occurrence is much delayed or even absent in thallic development (e.g. Geotrichum). Wall building is a preferable term to meristem which implies growth by cell division rather than within a single cell. See Minter et al. (TBMS 79: 75, 1982
[https://doi.org/10.1016/S0007-1536(82)80193-9], TBMS 80: 39, 1983
[https://doi.org/10.1016/S0007-1536(83)80163-6], TBMS 81: 109, 1983
[https://doi.org/10.1016/S0007-1536(83)80210-1]). Also see Anamorphic fungi. Return to top.
|
Fig. 41. Wall-building in relation to conidiogenesis. A, apical; B, diffuse; C, ring. After Minter et al. ( TBMS 80: 39, 1983). Return to top. |
|
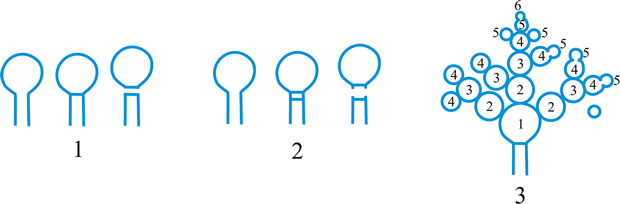
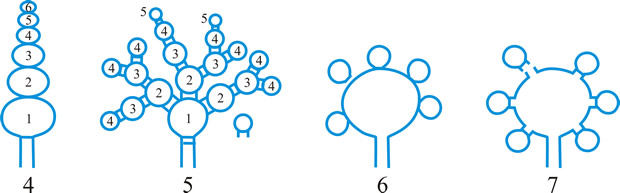
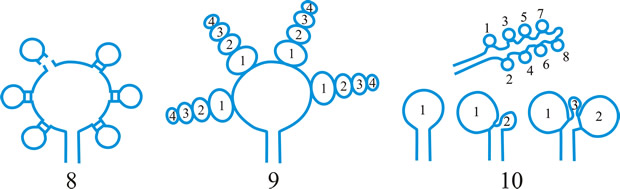
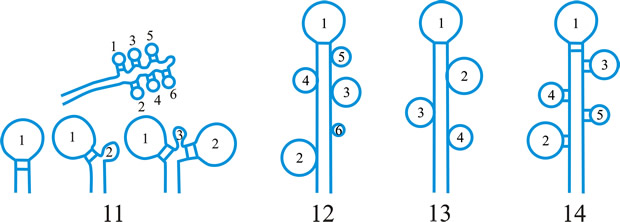
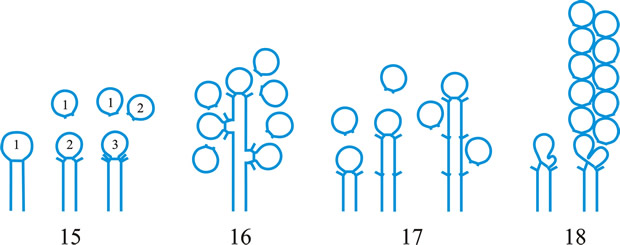
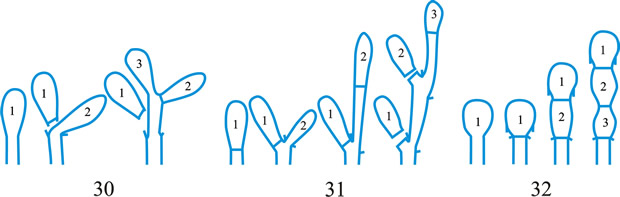
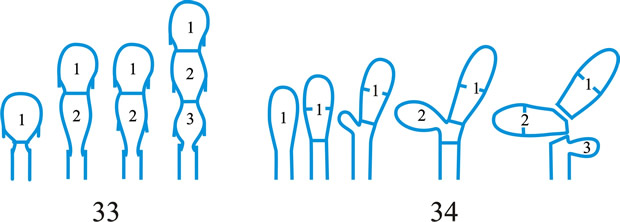
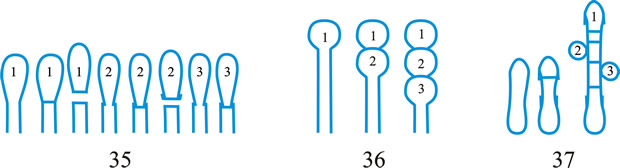
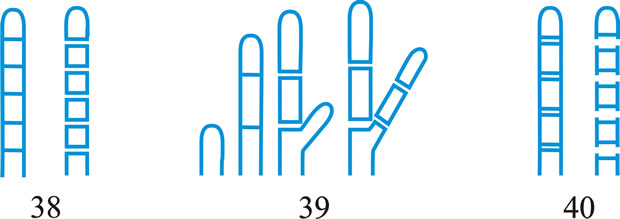
Fig. 1. Conidiogenous events (cc, conidiogenous cell). 1, conidial ontogeny holoblastic, one locus per cc, solitary conidia, delimited by one septum, maturation by diffuse wall-building, secession schizolytic, no proliferation of cc; 2, conidial ontogeny holoblastic, one locus per cc, solitary conidia, delimitation by two septa (or a separating cell), secession rhexolytic or by fracture of the cc, maturation by diffuse wall-building, no proliferation of cc; 3, conidial ontogeny holoblastic, apical wall-building random at more than one locus per cc and conidia becoming conidiogenous to form connected branched chains, each conidium delimited by one septum, maturation by diffuse wall-building, secession schizolytic, no cc proliferation; ...continued... |
|
Fig. 1 (continued); Conidiogenous events (cc, conidiogenous cell). 4, conidial ontogeny holoblastic, apical wall-building at one locus per cc and each conidium with one locus to form a connected unbranched chain, each conidium delimited by one septum, maturation by diffuse wall-building, secession schizolytic, no proliferation of cc; 5, conidial ontogeny holoblastic, apical wall-building randomly at more than one locus per cc and conidia becoming conidiogenous to form connected branched chains, each conidium delimited by two septa (or a separating cell), secession rhexolytic or by fracture of the cc, maturation by diffuse wall-building, no cc proliferation; 6, conidial ontogeny holoblastic, with localized apical wall-building simultaneously at different loci over the whole cc, each locus forming one conidium, delimited by one septum, maturation by diffuse wall-building, secession schizolytic, no cc proliferation; 7, conidial ontogeny holoblastic, with localized apical wall-building simultaneously at different loci on denticles over the whole cc, each locus forming one conidium, delimited by one septum, maturation by diffuse wall-building, secession by rupture of denticle, no cc proliferation; ...continued... |
|
Fig. 1 (continued); Conidiogenous events (cc, conidiogenous cell). 8, conidial ontogeny holoblastic, with localized apical wall-building simultaneously at different loci over the whole cc, each conidium delimited by two septa (or a separating cell), secession rhexolytic or by fracture of the cc, each locus forming one conidium, maturation by diffuse wall-building, no cc proliferation; 9, conidial ontogeny holoblastic, apical wall-building simultaneously at several loci per cc and conidia becoming conidiogenous to form connected branched chains, each conidium delimited by one septum, maturation by diffuse wall-building, secession schizolytic, no cc proliferation; 10, conidial ontogeny holoblastic, regularly alternating with holoblastic sympodial cc proliferation, maturation by diffuse wall-building, each conidium delimitated by one septum, secession schizolytic; ...continued... |
|
Fig. 1 (continued); Conidiogenous events (cc, conidiogenous cell). 11, conidial ontogeny holoblastic, regularly alternating with holoblastic sympodial cc proliferation, maturation by diffuse wall-building, each conidium delimited by two septa (or a separating cell), secession rhexolytic or by fracture of the cc; 12, conidial ontogeny holoblastic, each from apical or lateral loci, delimited by one septum, secession schizolytic, holoblastic cc proliferation sympodial or irregular, maturation by diffuse wall-building; 13, conidial ontogeny holoblastic, first from an apical locus, delimited by one septum, secession schizolytic, other conidia from lateral loci proceeding down the cc, maturation by diffuse wall-building; 14, conidial ontogeny holoblastic, first from an apical locus, each conidium delimited by two septa (or a separating cell), secession rhexolytic or by fracture of the cc, other conidia from lateral loci proceeding down the cc, maturation by diffuse wall-building. Return to top. |
|
Fig. 2. Conidiogenous events (cc, conidiogenous cell). 15, conidial ontogeny holoblastic, delimitation by one septum, schizolytic secession, maturation by diffuse wall-building, percurrent enteroblastic cc proliferation followed by conidial ontogeny by replacement apical wall-building, successive conidia seceding at the same level, sometimes in unconnected chains, collarette variable; 16, same as 15 but with several random or irregular conidiogenous loci to each cc; 17, conidial ontogeny holoblastic, delimitation by one septum, schizolytic secession, maturation by diffuse wall-building, percurrent enteroblastic cc proliferation followed by conidial ontogeny by replacement apical wall-building, successive conidia seceding at the same level, collarette variable, conidiogenous activity interspersed periodically with percurrent vegetative proliferation; 18, conidial ontogeny holoblastic, delimitation by one septum, schizolytic secession, maturation by diffuse wall-building, percurrent and sympodial enteroblastic cc proliferation followed by conidial ontogeny by replacement apical wall-building, successive conidia seceding at the same level, collarette variable; ...continued... |
|
Fig. 2. (continued); Conidiogenous events (cc, conidiogenous cell). 19, conidial ontogeny holoblastic, delimitation by one septum, schizolytic secession, maturation by diffuse wall-building, percurrent enteroblastic cc proliferation followed by conidial ontogeny by replacement apical wall-building, successive conidia seceding at progressively higher levels, sometimes in unconnected chains, collarette variable; 20, conidial ontogeny enteroblastic, delimitation by one septum, schizolytic secession, maturation by diffuse wall-building, outer wall of the cc remaining as a conspicuous collarette, percurrent enteroblastic cc proliferation followed by conidial enteroblastic ontogeny by replacement apical wall-building, successive conidia seceding at the same level, a succession of collarettes formed; 21, combination of 10, 12 and 19, where the sequences occur at random, irregularly or interchangeably; ...continued... |
|
Fig. 2. (continued); Conidiogenous events (cc, conidiogenous cell). 22, conidial ontogeny holoblastic with new inner walls constituting the conidia laid down retrogressively by diffuse wall-building, delimitation retrogressive, loss of apical wall-building followed by replacement ring wall-building at the base of the cc adding more retrogressively delimited conidia, the outer (original) cc wall breaks as a connected chain of conidia is formed, collarette variable, one locus per cc, secession schizolytic; 23, conidial ontogeny holoblastic, one locus per cc, first conidium delimited by one septum, maturation by diffuse wall-building, loss of apical wall-building, replaced by ring wall-building below the delimiting septum which produces conidia in a connected unbranched chain, secession schizolytic, no proliferation of cc; 24, conidial ontogeny holoblastic, simultaneous with minimal enteroblastic percurrent proliferation at the preformed pore in the outer cc wall, conidia solitary, delimited by one septum, secession schizolytic, maturation by diffuse wall-building, one locus per cc; 25, conidial ontogeny holoblastic, simultaneous with minimal enteroblastic percurrent proliferation at the preformed pore in the outer cc wall, conidia solitary, delimited by one septum, secession schizolytic, maturation by diffuse wall-building, after one conidium formed extensive enteroblastic percurrent proliferation by apical wall-building occurs until the next apical locus is formed; ...continued... |
|
Fig. 2. (continued); Conidiogenous events (cc, conidiogenous cell). 26, same as 24 but with holoblastic sympodial proliferation of the cc with conidiogenesis occurring between loci; 27, same as 24 but with several conidiogenous loci produced in the apical cc and laterally below septa in other ccs constituting the conidiophore; 28, same as 24 but several loci to each cc and first and subsequent conidia becoming conidiogenous by apical wall-building to form unbranched connected chains; more than one locus to a conidium will produce branched chains; 29, same as 24 but first conidium becoming conidiogenous by apical wall-building to form an unbranched connected chain. Return to top. |
|
Fig. 3. Conidiogenous events (cc, conidiogenous cell). 30, conidial ontogeny holoblastic, delimitation by one septum, maturation by apical and diffuse wall-building, secession schizolytic and coincident with enteroblastic sympodial cc proliferation below the previous locus; subsequent conidia formed similarly but with holoblastic sympodial cc proliferation; 31, conidial ontogeny holoblastic, delimitation by one septum, maturation by apical and diffuse wall-building, secession schizolytic and coincident with enteroblastic sympodial cc proliferation below the previous conidiogenous locus, the sequence giving geniculate conidiophores; 32, conidial ontogeny holoblastic, with new inner walls continuous with all conidia laid down by diffuse wall-building, delimitation by one septum, loss of apical wall-building followed by replacement continuous ring wall-building immediately below delimiting septum, the outer cc wall breaks between the first conidium and the cc to produce a variable collarette, followed by alternation of holoblastic conidial ontogeny by ring wall-building giving connected chains of conidia, maturation by diffuse wall-building, retrogressive delimitation, secession schizolytic; ...continued... |
|
| Fig. 3. (continued); Conidiogenous events (cc, conidiogenous cell). 33, conidial ontogeny holoblastic with new inner walls laid down by diffuse wall-building, delimitation by one septum, loss of apical wall-building followed by replacement ring wall-building immediately below delimiting septum, the outer cc wall breaks between the first conidium and the cc to produce a variable collarette, subsequent conidia formed by new inner walls for each conidium by ring wall- building giving connected chains of conidia, maturation by diffuse wall-building, retrogressive delimitation, secession schizolytic; 34, conidial ontogeny holoblastic, delimitation by one septum, secession schizolytic, enteroblastic sympodial cc proliferation below the previous locus and delimiting septum, the second and subsequent conidia formed from proliferations and delimited retrogressively, cc reduced in length with each conidium formed;...continued... |
|
| Fig. 3. (continued); Conidiogenous events (cc, conidiogenous cell). 35, conidial ontogeny holoblastic, maturation by diffuse wall-building, delimitation by one septum, secession schizolytic, enteroblastic percurrent cc proliferation with retrogressive delimitation of next conidium, producing unconnected chains of conidia, the cc reduced in length with each conidium formed; 36, conidial ontogeny holoblastic, delimitation by one septum with loss of apical wall-building but replaced by diffuse wall-building below the previous conidium to form the next conidium which is retrogressively delimited giving an unconnected chain of conidia, secession schizolytic, cc reduced in length with each conidium formed; 37, conidial ontogeny holoblastic, delimitation by one septum with loss of apical wall-building, replaced by ring wall-building below the delimiting septum, outer wall of first conidium and cc breaks, followed by enteroblastic percurrent proliferation by ring wall-building, succeeding conidia holoblastic, delimited laterally and retrogressively, secession schizolytic, several loci per cc; ...continued... |
|
| Fig. 3. (continued); Conidiogenous events (cc, conidiogenous cell). 38, conidial ontogeny holothallic, ccs formed by apical wall-building coincident with conidial ontogeny, random delimitation by one septum at each end, no maturation during conidiogenesis, secession randomly schizolytic; 39, conidial ontogeny holothallic, ccs formed by apical wall-building coincident with conidial ontogeny, random delimitation by one septum at each end, no maturation during conidiogenesis, secession randomly schizolytic, cc proliferation holoblastic, irregular or sympodial, constituent cells conidiogenous; 40, same as 38 but conidial delimitation by two septa or separating cells at each end, secession rhexolytic; ...continued... |
|
Fig. 3. (continued); Conidiogenous events (cc, conidiogenous cell). 41, conidial ontogeny holothallic, ccs formed in association with clamp connexions, random delimitation by septa in cc and the backwardly directed branch in the clamp connexion, maturation by diffuse and localized apical wall-building, secession randomly schizolytic, individual conidia comprised of part of the preceding and following clamp connexions; 42, conidial ontogeny holoblastic by simultaneous apical wall-building in adjacent cells, delimitation by septa in each of these cells, maturation by diffuse wall-building, secession simultaneous, multicellular, schizolytic, no cc proliferation; 43, conidial ontogeny holoblastic by simultaneous apical wall-building in adjacent cells, delimitation by septa in each of these cells, maturation by diffuse wall-building, followed by replacement apical wall-building in conidia to form additional conidia in connected chains, secession simultaneous, multicellular, rhexolytic, no cc proliferation. Return to top. |
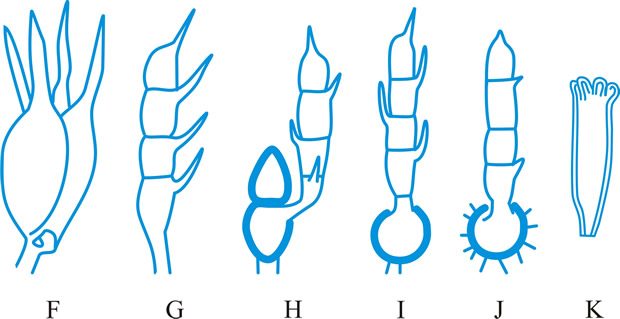
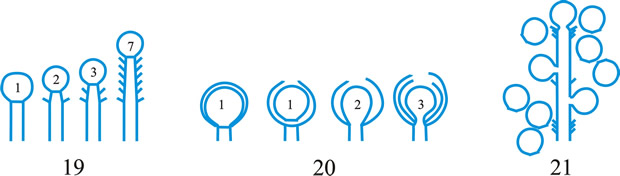
conidioma (pl. -ata), a specialized multihyphal, conidia-bearing structure (Kendrick & Nag Raj in Kendrick (ed.), The whole fungus 1: 51, 1979
[https://doi.org/10.1002/fedr.19800910312]). See acervulus, pycnidium, sporodochium, synnema (all obsol. nouns, but used adjectivally, e.g. acervular conidioma). See Fig. 13. Cf. conidiophore. Return to top.
|
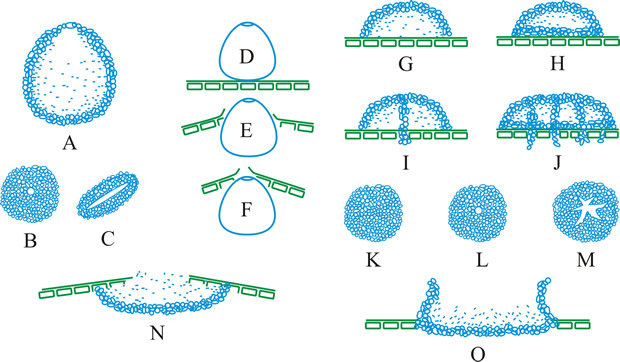
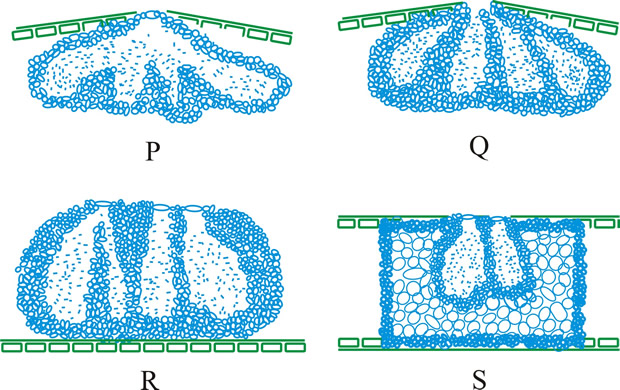
Fig. 13. Conidiomatal types. A- F, pycnidial; B, dehiscence by a central circular ostiole; C, dehiscence by a longitudinal ostiole (raphe); D, superficial; E, semi-immersed; F, immersed; G- M, pycnothyrial; G with upper wall only; H with upper and lower walls; I, with a central supporting column; J, multilocular with several supporting columns; K, dehiscence from the margin; L, dehiscence by a central ostiole; M, dehiscence by irregular fissures; N, acervular; O, cupulate; ...continued... |
|
Fig. 13. (continued) Conidiomatal types. P, convoluted immersed; Q, multilocular, immersed; R, multilocular, superficial; S, pseudostromatic. Return to top. |
Differentiation in ascomata
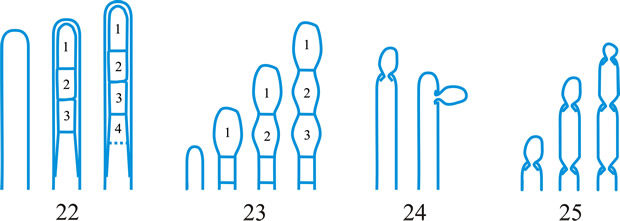
ascus (pl. asci), term introduced by Nees (Syst. Pilze: 164, 1817) for the typically sac-like cell (first figured in Pertusaria by Micheli in 1729; q.v.) characteristic of Ascomycota (q.v.), in which (after karyogamy and meiosis) ascospores (generally 8) are produced by ‘free cell formation’ (see Fig. 5 in Chapter 3 plus associated text, AND Fig. 14A in Chapter 9). Asci vary considerably in structure, and work in the last two decades has shown previous separation into only 2-3 categories (e.g. bitunicate, prototunicate, unitunicate) to be an over-simplification. Sherwood (1981) illustrated 9 main types distinguishable by light microscopy (reproduced on p. 36 of edn 7 of this Dictionary): prototunicate, bitunicate, astropalean, annellate, hypodermataceous, pseudoperculate, operculate, lecanoralean, and verrucarioid. Eriksson (1981) distinguished 7 types of dehiscence in bitunicate asci with an ectotunica and distinct endotunica (see p. 37 of edn 7). These classifications mask a much wider range of variation; Bellemere (1994) recognized 3 predehiscence types and 11 dehiscence categories (Fig. 4). The details of the asci are stressed in ascomycete systematics, esp. in lichen-forming orders where reactions with iodine are emphasized (q.v.) (Hafellner, 1984). Return to top.
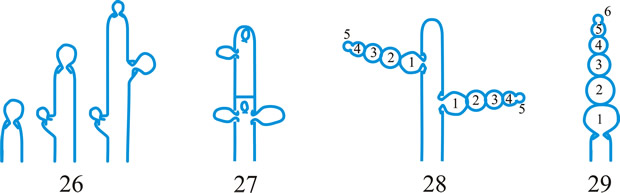
Bitunicate asci with two functional wall layers; those splitting at discharge (fissitunicate; ‘jack-in-the-box’) had been correlated with an ascolocular ontogeny by Luttrell (1951). Reynolds (1989) critically examined this paradigm and found the term to be applied to different ascus types and that an exclusive link to ascostromatic fungi could not be upheld; he also introduced the term extenditunicate for asci which extend without any splitting of the wall layers (Reynolds, Cryptog. Mycol. 10: 305, 1989). Much variation depends on the modifications in the various wall layers, especially the thickness of the walls and the c and d layers, and the details of apical differentiation (Bellemere, 1994) (Fig. 5). Caution is needed in comparing ascus staining reactions (see iodine) and structures in the absence of ultrastructural data. For terms used to describe the various structures see Fig. 5. Return to top.
Also encountered are ascus crown (annular thickenings in Phyllachora), and ascus plug (thickening in the apex through which the spores are forcibly discharged). Return to top.
|
Fig. 4. I. Predehiscence stage of asci. a, protruding ascus; b, ascus wall becoming thinner; c, change in apical structure; d, ascus liberation. ...continued... |
|
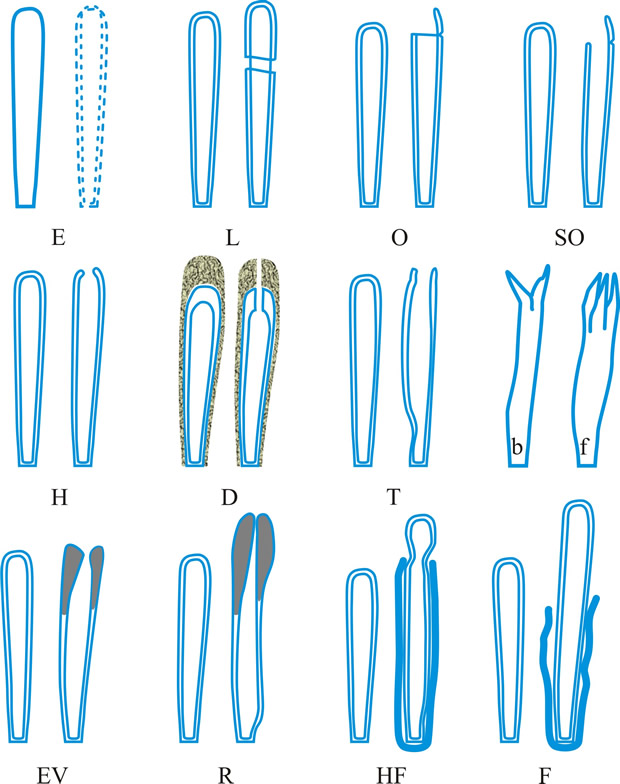
Fig. 4. (continued); II Dehiscence stage of asci; evanescent ascus ( E); rupture of lateral wall ( L); subapical rupture ( O, operculate, and SO, suboperculate dehiscence); rupture by apical wall without extrusion ( H, pore-like dehiscence); D, Dactylospora-type; T, Telochistes-type, extenditunicate ( b= bivalve, f, fissurate variants); rupture with extrusion ( EV, eversion; R, rostrate; HF, hemifissitunicate; F, fissitunicate). After Bellemére, in Hawksworth (ed.) Ascomycete Systematics: 111, 1994. Return to top. |
|
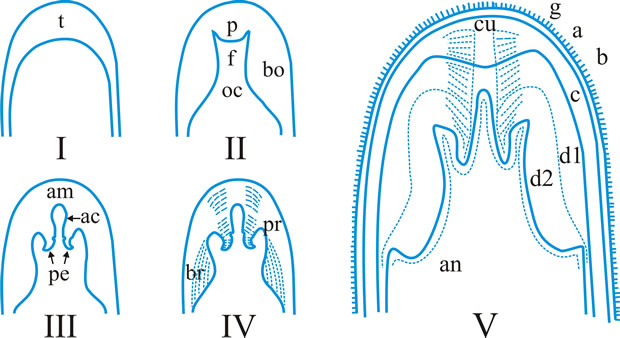
Fig. 5. I- V. Ascus apex components. ac, axial canal; am, axial mass; bo, bourrelet; br, ring in bourrelet; f, furrow; oc, ocular chamber; p, plug; pe, pendant; pr, rings in the plug and pendant; t, tholus; V, ascus apex structure. a, 'a' layer; an , apical nasse; b, 'b' layer; c, 'c' layer; d1 and d2, sublayers of the 'd' layer. After Bellemére, in Hawksworth (ed.) Ascomycete Systematics: 111, 1994. Return to top. |
tissue types, Korf distinguished the types of hyphal tissues in discomycetes as different textura’s and this is now applied to all ascomycetes and coelomycetes. Tissue (textura) types (from Korf, Sci. Rep. Yokohama nat. Univ. II 7: 13, 1958; which is derived from Starbäck, 1895). See also Dargan (Nova Hedw. 44: 489, 1987; Xylariaceae), plectenchyma. See Fig. 38. Return to top.
|
Fig. 38. Hyphal tissue (textura) types (Korf, 1958). A, textura globulosa; B, textura angularis; C, textura prismatica; D, textura intricata; E, textura epidermoidea; F, textura oblita; G, textura porrecta. Return to top. |
Differentiation in basidiomata
dolipore septum, a septum of a dikaryotic basidiomycete hypha which flares out in the middle portion forming a barrel-shaped structure with open ends as shown by electron microscopy; see Markham (MR 98: 1089, 1994; review
[https://doi.org/10.1016/S0953-7562(09)80195-0]), Moore (in Hawksworth (ed.), Identification and characterization of pest organisms: 249, 1994
[ISBN-10: 0851989047, ISBN-13: 978-0851989044]; summary diagram in Fig. 16 below), Moore & McAlear (Am. J. Bot. 49: 86, 1962
[https://doi.org/10.2307/2439393]); septal pore swelling; cf. parenthesome. Return to top.
|
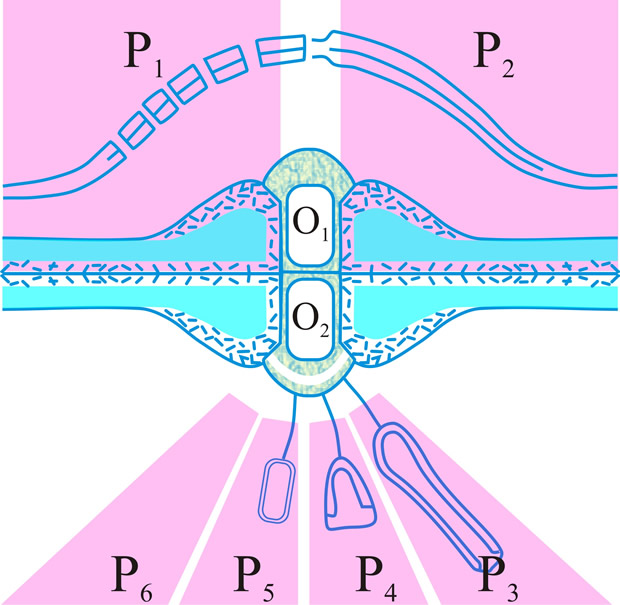
Fig. 16. Summary diagram of the several forms of the dolipore/parenthesome (d/p) septum in the Basidiomycota. The outer part of the mushroom-shaped occlusions can appear either as a granule (O1) or a striated band (O2); the parenthesomes can be regularly perforate (P1), imperforate (P2), vesiculate (P3-5), or absent (P6). Particular taxa are generally associated with distinct d/p combinations: O1/P1, subclass Homobasidiomycetidae; O1/P2, polypore tribes Phellineae and Hirschioporae, Heterobasidiomycetidae, orders Auriculariales, Dacrymycetales and Tulasnellales, and suborder Exidiineae (note the central thin spot in which the intervening line in the double membrane morphology is absent); O2/P3,4,6, suborder Tremellineae; O2/P5, Wallemia sebi. Adapted from Moore, in Hawksworth (ed.) (1994; 252
[ISBN-10: 0851989047, ISBN-13: 978-0851989044]). Return to top. |
basidium (pl. -ia), (1) the cell or organ, diagnostic for basidiomycetes, from which, after karyogamy and meiosis, basidiospores (generally 4) are produced externally each on an extension (sterigma, q.v.) of its wall (Fig. 9); (2) a conidiophore or phialide (obsol.). Return to top.
The confused terminology applied to basidia (sense 1) and their parts has been traced by Clémençon (Z. Mykol. 54: 3, 1988
[URL:
https://www.dgfm-ev.de/publikationen/artikelarchiv/die-basidie/download]) and is analysed by Talbot (TBMS 61: 497, 1973
[https://doi.org/10.1016/S0007-1536(73)80119-6]) whose recommended usage (basically that of Donk) and synonymy is adopted in the series of definitions which follow (see Fig. 10): pro-, the morphological part or developmental stage of the basidium in which karyogamy occurs; primary basidial cell; pro-basidial cyst; hypo- (Martin) p.p.; teliospore of Uredinales. meta-, (1) the morphological part or developmental stage in which meiosis occurs; hypo- (Martin) p.p.; epi- (Martin) p.p.; promycelium of Uredinales. (When the whole meta- includes pro- remnants the distal and functional part may be distinguished as a pario- (Talbot, 1973).) (2) See proto- below, holo-, a basidium (e.g. of Agaricus) in which the meta- is not divided by primary septa (see septum) but may become adventitiously septate (see septum) (Talbot, Taxon 17: 625, 1968). A holo- may be a sticho-, cylindrical, with nuclear spindles longitudinal and at different levels, or a chiasto-, clavate, with nuclear spindles across the basidium and at the same level (see Fig. 9C, D). phragmo-, a basidium in which the meta- is divided by primary septa, usually cruciate (e.g. Tremella) or transverse (e.g. Auricularia) (Talbot, 1968
[https://doi.org/10.2307/1218002]). Return to top.
Among other terms applied to basidia are: apo-, one with non-apiculate spores borne symmetrically on the sterigmata and not forcibly discharged (Rogers, Mycol. 39: 558, 1947
[https://doi.org/10.2307/3755195]); auto-, one with spores borne asymmetrically and forcibly discharged; endo-, one developing within the basidioma, as in gasteromycetes; epi-, Martin’s term for protosterigma, see sterigma; hetero-, a basidium of the Heterobasidiomycetes, usually a phragmo-; homo-, a basidium of the Homobasidiomycetes, usually a holo-; hypo-, = pro- (Donk), meta- (Martin); (of Septobasidium), basidium (Martin); pleuro-, one relatively broad at the base and with bifurcated spreading ‘roots’, as in Pleurobasidium (Donk); proto-, a primitive basidium; the opposite of meta- in the sense of changed or degenerate basidium; repeto-, see Chadefaud (Rev. mycol. 39: 173, 1975); sclero-, the thick-walled, encysted, gemma-like pro- of the Uredinales (teliospore) and the Auriculariales (Janchen, 1923). See also Wells & Wells (Basidium and basidiocarp evolution, cytology, function and development 1982
[https://doi.org/10.1007/978-1-4612-5677-9]). Return to top.
|
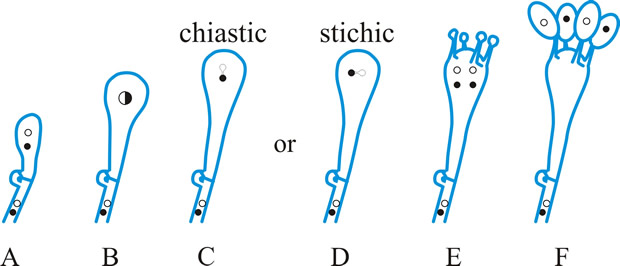
Fig. 9. Basidiospore development (diagrammatic). A- E meiosis ( C, chiastic, and D, stichic). E, diploid probasidium; F, basidium (metabasidium) with four basidiospores on sterigmata. Return to top. |
|
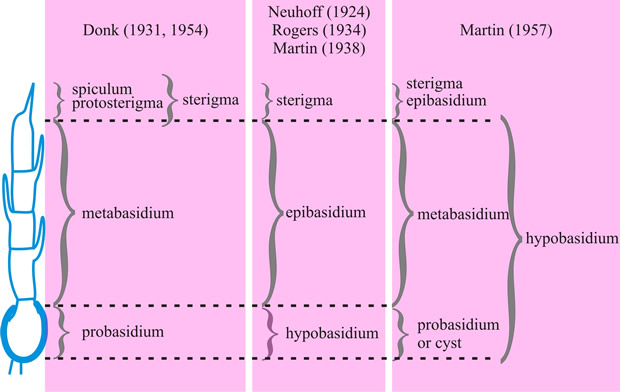
Fig. 10. Basidium terminology, to compare the terminology of different authors, illustrated with reference to the Septobasidium-type (Talbot, 1973
[ https://doi.org/10.1016/S0007-1536(73)80119-6]). Note that in this extreme case the metabasidium of Donk coincides with that of Martin. Return to top. |
|
Fig. 11. Basidial types. A- E, holobasidial ( A, B, apobasidial; C, D, E, autobasidial). A, Lycoperdales; B, Tulostomatales; C, Agaricales; D, Dacrymycetales; E, Tulasnellales. ...continued... |
|
Fig. 11. (continued; Basidial types. F- K, phragmobasidial ( F, G, Basidiomycetes; H, I, Teliomycetes; J, K, Ustomycetes). F, Tremellales; G, Auriculariales; H, Uredinales; I, Septobasidiales; J, Ustilaginales; K, Cryptobasidiales. Return to top. |
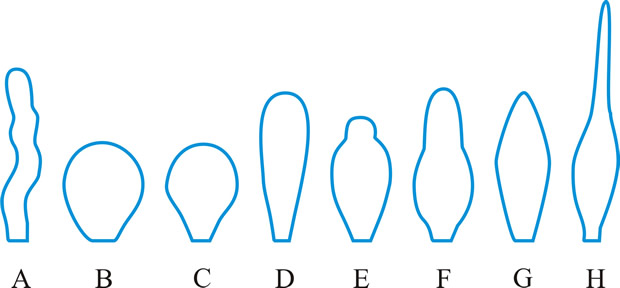
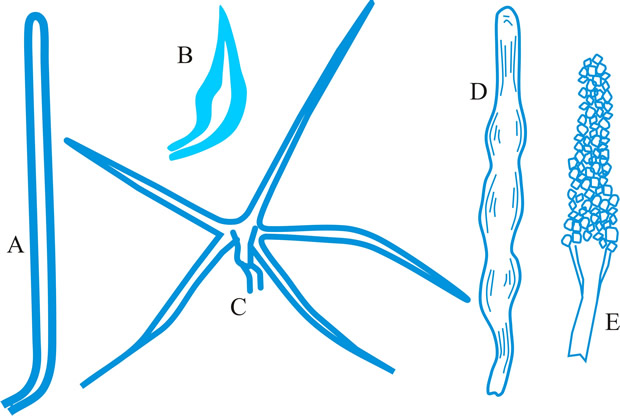
cystidium (pl. -ia), a sterile body, frequently of distinctive shape, occurring at any surface of a basidioma, particularly the hymenium from which it frequently projects (Fig. 15). Cystidia have been classified and named according to their: (1) origin: hymenial - (tramal -), originating from hymenial (tramal) hyphae; pseudo-, derived from a conducting element, filamentous to fusoid, oily contents, embedded not projecting; coscino-, see coscinoid; skeleto-, the apical part of a skeletal hypha (frequently ± inflated) projecting into or through the hymenium; false seta; macro -, arising deep in the trama in Lactario-Russulae; hypho-, hypha-like, derived from generative hyphae. (2) position: (first by Buller, 2): on the pileus surface (pileo-, dermato-, Fayod); at the edge (cheilo-), side (pleuro-), or within (endo-) a lamella; on the stipe (caulo-). (3) form: lepto-, smooth, thin-walled; lampro-, thick-walled, with or without encrustation (setiform lampro-, awl-shaped, wall pigmented; asteroseta, a radially branched lampro-; microsclerid, a versiform, endolampro-; lyo-, cylindrical to conical, very thick-walled, apex abruptly thin-walled, not encrusted, hyaline, as in Tubulicrinis (Donk, 1956); monilioid gloeo-, (torulose gloeo- (Bourdot & Galzin, 1928)); monili-form paraphysis (Burt, 1918); pseudophysis; schizo- (Nikolayeve, 1956, 1961)), monilioid, frequently with a beaded apex (as in Hericiaceae and Corticiaceae). (4) contents: gloeo-, thin-walled, usually irregular, contents hyaline or yellowish and highly refractile; chryso-, like lepto- but with highly staining contents; hypo-, (Larsen & Burdsall, Mem. N.Y. bot. Gdn 28: 123, 1976); oleo-, having an oily resinous exudate; pseudo-, see (1) above. See also hyphidium, seta. Reviews of cystidia include Romagnesi (Rev. Mycol., Paris 9 (suppl.): 4, 1944), Talbot (Bothalia 6: 249, 1954), Lentz (Bot. Rev. 20: 135, 1954
[URL:
http://www.jstor.org/stable/4353512]), Smith (in Ainsworth & Sussman (eds), The Fungi 2: 151, 1966
[URL:
http://krishikosh.egranth.ac.in/handle/1/2061852]), Price (Nova Hedw. 24: 515, 1975; types in polypores). Return to top.
|
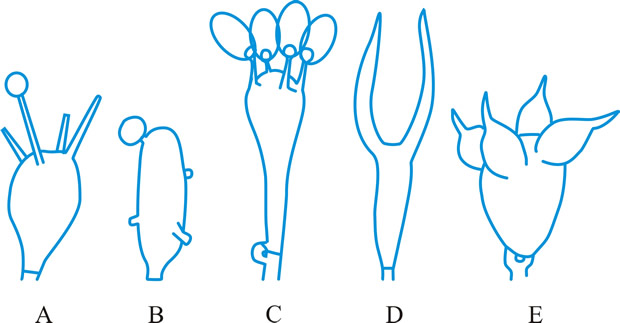
Fig. 15. Cystidia. A, hyphoid ( Collybia); B, globose ( Agaricus); C, pyriform ( Agaricus); D, clavate ( Inocybe); E, utriform ( Psathyrella); F, lageniform ( Pholiota); G, fusoid ( Psathyrella); H, lanceolate ( Hypholoma); ...continued... |
|
Fig. 15. (continued); Cystidia. I, capitate ( Hyphoderma), J, tibiiform ( Galerina); K, lecythiform ( Conocybe); L, urticoid ( Naucoria); M, metuloid ( Lentinus), N, gloeocystidium ( Gloeocystidiellum); O, macrocystidium ( Russula); P, chrysocystidium ( Stropharia). Not to scale. Return to top. |
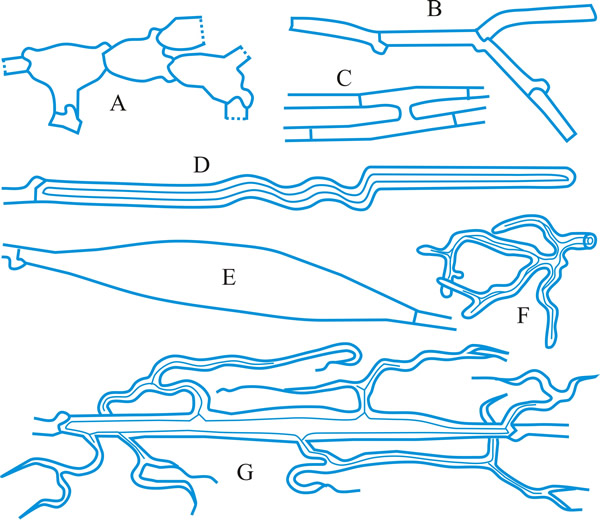
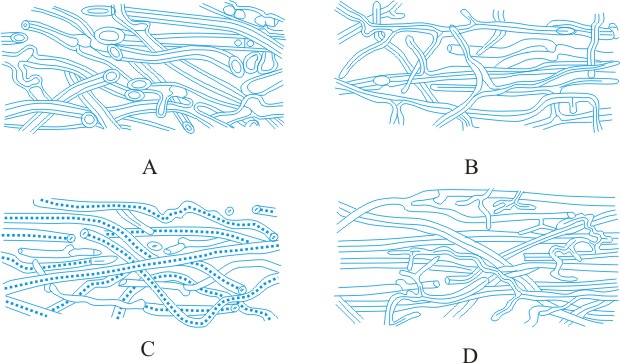
Hyphal analysis. A procedure by which the development and structure of basidiomata can be investigated, providing important taxonomic criteria. Three main types of hyphal systems of increasing complexity were recognized by Corner (TBMS 17: 51, 1932
[https://doi.org/10.1016/S0007-1536(32)80026-4]) (Figs. 19, 20 below): monomitic, having hyphae of one kind (generative hyphae which are branched, septate, with or without clamp-connections, thin- to thick-walled, and of unlimited length; they give rise both to other hyphal types and to the hymenium) (Teixeira, Mycol. 52: 30, 1961
[https://doi.org/10.2307/3756248]; gen. hyphae of polypores); dimitic, having hyphae of two kinds (generative and skeletal hyphae which are thick-walled, aseptate, and of limited length, with thin-walled apices, generally unbranched but when terminal they can develop arboriform branching or taper) or generative and binding (see below); trimitic, having hyphae of three kinds (generative, skeletal and binding (or ligative) hyphae which are aseptate, thick-walled, much branched, either Bovista-type with tapering branches or coralloid; they bind the skeletal and generative hyphae together). In Polyporaceae and Lentinaceae, intercalary skeletal hyphae can give rise to ligative branching, the entire element being termed a skeleto-binding cell (Corner, 1981)
or skeleto-ligative hypha (Pegler, 1973, The polypores: with keys to
world genera and British species. Bulletin of the British
Mycological Society, 7 (supplement): pp. 3-43 [https://doi.org/10.1016/S0007-1528(73)80020-3];
1983, The genus Lentinus: a world monograph [ISBN: 0112426271,
9780112426271]). Corner also recognizes sarcodimitic (in which the skeletal hyphae are replaced by thick-walled, long, inflating fusiform elements) and sarcotrimitic (in which the generative hyphae give both thick-walled inflated elements similar to binding hyphae but septate) types. Return to top.
Most soft and fleshy basidiomata are monomitic, with hyphae which are generally inflated (most agaricoid and clavarioid fungi). Hard and tough basidiomata may be monomitic with the generative hyphae developing thickened walls, dimitic, with skeletal hyphae (e.g. Phellinus spp.) or (esp. when perennial) trimitic (e.g. Fomes, Ganoderma, Microporus xanthopus). Every species has a well-defined and constant construction, which is maintained regardless of changes in the external morphology of the basidioma due to environmental conditions, hence the importance of hyphal analysis in taxonomy. Lit.:
Corner (Annals of Botany, 46: 71, 1932,
Phytomorphology 3: 152, 1953, Beihefte zur
Nova Hedwigia, 75: 13, 1983, 78:
13, 1984), Cunningham (New Zealand Journal of Science and Technology,
28(A): 238, 1946, TBMS 37:
44, 1954 [https://doi.org/10.1016/S0007-1536(54)80066-0]),
Lentz (Botanical Review, 20: 135, 1954),
Talbot (Bothalia, 6: 1, 1951 [https://doi.org/10.4102/abc.v6i1.1681]). Return to top.
|
Fig. 19. Hyphal types. A, inflated generative hyphae; B, non-inflated generative hyphae with clamp connexions; C, generative hyphae without clamp connexions; D, unbranched skeletal hypha; E, sarco-hypha; F, highly branched ligative (binding) hypha; G, skeleto-ligative hypha. Not to scale. Return to top. |
|
Fig. 20. Hyphal systems. SSee Pegler (1973,
Bulletin of the British Mycological Society, 7
(supplement): pp. 3-43 [ https://doi.org/10.1016/S0007-1528(73)80020-3]). A, monomitic hyphal system, with thick-walled generative hyphae; B, dimitic hyphal system, with generative and ligative (binding) hyphae; C, dimitic hyphal system, with generative and skeletal hyphae; D, trimitic hyphal system, with generative, skeletal and ligative hyphae. Return to top. |
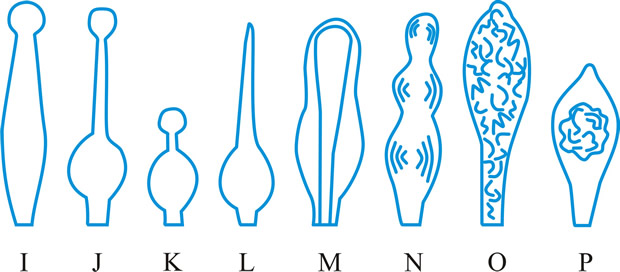
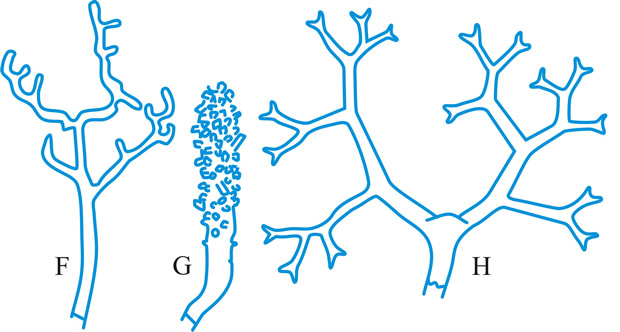
hyphidium (pl. -ia), (paraphysis, pseudoparaphysis, paraphysoid, dikaryoparaphysis, and pseudophysis sensu Singer (1962) are syn. or near syn.), a little, or strongly, modified terminal hypha in the hymenium of hymenomycetes (Fig. 21 below). Donk (Persoonia 3: 229,
1964 [URL:
http://www.repository.naturalis.nl/record/532067]) distinguished; haplo- (simple -), unmodified, unbranched or little branched; dendro- (dendrophysis), irregularly strongly branched; dicho- (dichophysis), repeatedly dichotomously branched; acantho- (acanthophysis; bottle-brush paraphysis (Burt, 1918)), having pin-like outgrowths near the apex; in Corticiaceae may be botryose, clavate, coralloid, or cylindrical. Cf. cystidium. Return to top.
|
Fig. 21. Hyphidia. A, setal hypha ( Phellinus); B, seta ( Inonotus); C, asteroseta ( Asterostroma); D, gloeo-hypha ( Gloeocystidiellum); E, encrusted ( Peniophora); ...continued... |
|
Fig. 21. (continued); Hyphidia. F, dendrohyphidium ( Cytidia); G, acanthohyphidium ( Aleurodiscus); H, dendrohyphidim ( Vararia). Not to scale. Return to top. |
References
Kirk, P.M., Cannon, P.F., Minter, D.W. &
Stalpers, J.A. (2008). Dictionary of the Fungi,
10th edn. Wallingford: CABI Publishing. ISBN-10: 0851998267, ISBN-13:
978-0851998268. Kindle edition:
ASIN: B00K7ANXJI.
Ulloa, M. & Hanlin, R.T. (2012).
Illustrated Dictionary of Mycology, Second Edition.
APS Press. ISBN: 978-0-89054-502-7. DOI:
https://doi.org/10.1094/9780890545027.
Return to top.
Updated July, 2018
|

types.jpg)